Electronic Structure of the Atom
and
Chemical Bonding
Structure of the Atom
In general chemistry, we focus on three subatomic particles that make up
all atoms. These are the electron, proton, and neutron. We
will not find it necessary to sort out the "particle zoo" that is the realm
of particle physics. We will find that the chemistry we look at can
be explained by the 3 particles mentioned above, and that most of the time,
we focus our attention on the electron.
In the atom, the protons and neutrons are found in the center to form
what we call the nucleus, while the electrons are found moving around in
the space surrounding the nucleus. Each proton is said to carry 1
unit of positive charge, while, neutrons, as the name suggests, are electrically
neutral. The electrons each carry one unit of negative charge.
Thus, the nucleus is positively charged, and the negative charge is distributed
around the nucleus to fill what we might call the volume of the atom.
The element to which an atom belongs is characterized by the number
of protons in its nucleus. This is called the atomic number.
Two elements can belong to the same element and yet differ in the number
of neutrons. These are called isotopes. Figure 1 shows the
the arrangement of the particles in hydrogen atoms.
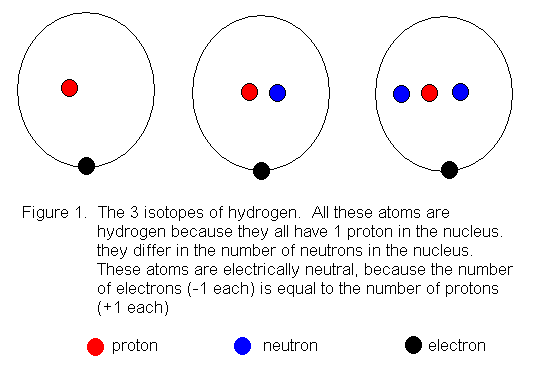
The above drawings are called "Bohr Model Drawings" because they suggest
that the electron orbits around the nucleus of the atom much like the planets
of our solar system orbit around the sun, as the Danish physist Niels Bohr
proposed. We now know that the motion of the electrons around the
nucleus is not so predictable. We can not precisely locate the electrons,
but we must instead speak of the probability of finding an electron within
a particular region of space. Nevertheless, there is still much we
can explain proceeding from nothing more than an "old" Bohr model, so I
have decided to develop as much of the theory as possible using this model
(because it is simpler) and then develop the quantum mechanical description
as it is needed. The "Lewis Diagrams" -- which are still frequently
used in general chemistry, and which we shall learn how to draw -- are
essentially just abbreviated Bohr Model drawings.
Hydrogen atoms are the simplest atoms, having only a single proton in
the nucleus. Other elements, of course, have more than one proton,
and generally consist of more than one nuclide -- that is, they exist as
isotopes. For simplicity, however, I will only draw one of the element's
nuclides. Our principle focus here is going to be on the arrangement
of the electrons, not the number of isotopes, or the number of neutorns
in those isotopes.
In the Bohr model, the electrons are pictured as orbiting around the
nucleus like planets around the sun. But unlike our solar system,
where each planet has its own orbit, in the Bohr model of the atom, more
than one electron can share the same "orbit". These "orbits" are
usually called "shells". The first shell can hold a maximum of 2
electrons, so we can draw an electrically neutral helium atom (atomic number
2) like this:
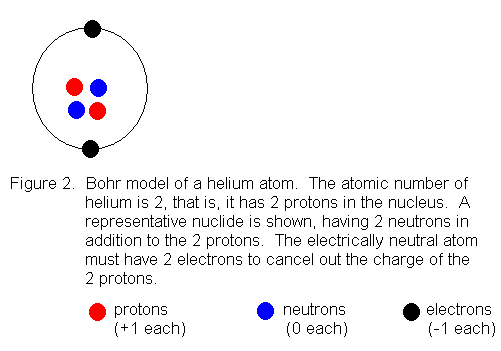
When we get to the element lithium (atomic number 3) we need a second
shell to hold the third electron, since the first shell can only hold 2
electrons. Shown below is a Bohr model drawing of a lithium atom
with a mass number of 7.
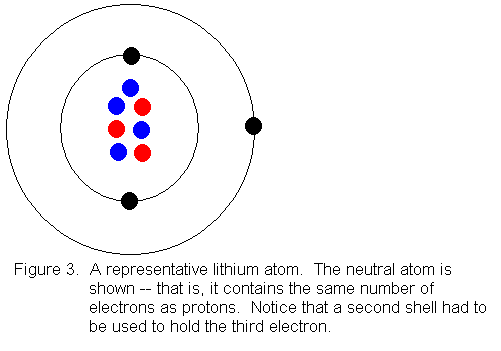
At this point, you're probably wondering how to know when to open up
another shell to contain additional electrons. There are two shell
capacities we need to be concerned with. One is the absolute maximum
capacity, and the other is the valence shell capacity. The absolute
maximum capacity of a shell is, of course, the largest number of electrons
that can ever fit in that shell at the same time. The valence shell
capacity is normally 8, but is 2 for the first shell. The valence
shell is the highest (that is, most distant from the nucleus) shell that
contains electrons. In figures 1 and 2, only the first shell was
used, so it was the valence shell. All the other shells were empty,
so were not drawn. Likewise, in Figure 3, shells 3 and up are not
drawn, because they are empty. The valence shell in the lithium atom
is the second shell. We number the shells from the inside out, so
the shell closest to the nucleus is shell number 1, the next one is shell
number 2 and so on. We usually use the variable n to represent the
shell number, and speak of, for example, the n=1 shell (read "n equals
one shell"). With this as background, I present the following table
of electron capacities:
| SHELL NUMBER (n) |
ABSOLUTE MAX CAPACITY |
VALENCE CAPACITY |
| 1 |
2 |
2 |
| 2 |
8 |
8 |
| 3 |
18 |
8 |
| 4 |
32 |
8 |
| n |
2n2 |
8 |
Notice that the higher the shell, the larger its absolute maximum electron
capacity. However, when a shell is a valence shell, it normally holds
no more than 8 electrons, regardless of the capacity it would otherwise
have. This is referred to as the octet rule. A notable exception
to the octet rule is the first shell. Its absolute maximum electron
capacity is only 2 electrons, which applies also when it is a valence shell.
(We might call the situation for the first shell, the duet rule.)
But the octet rule has an even broader application than merely pointing
out that valence shells are normally full when they contain 8 electrons.
As we will see, atoms are most stable when they have full valence shells,
and the resulting tendency to obtain such arrangements is the driving force
behind chemical reactions, and explains why elements combine in the proportions
they do.
The table above gives the absolute maximum electron capacities for the
first 4 shells. The last entry in the table is a generic one, which
shows how the other entries in the table were calculated. The absolute
maximum electron capacity is twice the square of the shell number.
Try taking the shell number, squaring it, and multiplying by 2, and you
will indeed reproduce the entries shown as absolute maximum capacities
for shells 1 through 4.
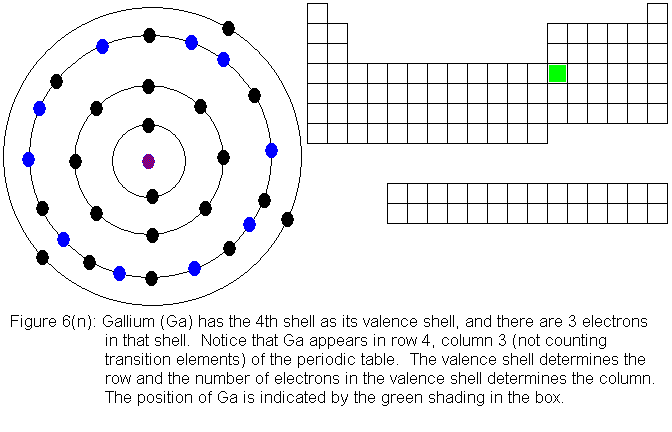
It is primarily the number of electrons in an atom's valence shell that
determines the atom's chemical properties. The number of electrons
the atom already has (in its valence shell) determines how it will obtain
a full shell. Thus, two different atoms (that is, belonging to different
elements) will behave similarly if they have the same number of valence
electrons.
The periodic table is a listing of the elements based on similarity
of chemical properties. The Russian chemist Dmitri Mendeleev was
the first to publish what has evolved into our modern periodic table.
Mendeleev listed the known elements in order of increasing atomic weight.
Rather than listing them in a continuous row, he broke the row into columns
so that elements with similar properties fell in the same column.
His assumption at the time was that when listed in order of increasing
atomic weight, elements with similar chemical properties would occur at
regular intervals. This was referred to as the periodic law.
In order to make this work, however, Mendeleev found he sometimes had to
leave gaps in his table. He had to do this to make elements with
similar properties fall in the same column. Consider, for example,
the following excerpt of the table as it might have appearred in his day.
|
I |
II |
III |
IV |
V |
VI |
VII |
| 1 |
H 1 |
|
|
|
|
|
|
| 2 |
Li 7 |
Be 9 |
B 11 |
C 12 |
N 14 |
O 16 |
F 19 |
| 3 |
Na 23 |
Mg 24 |
Al 27 |
Si 28 |
P 31 |
S 32 |
Cl 35 |
| 4 |
K 39 |
Ca 40 |
? ?? |
Ti 48 |
V 51 |
Cr 52 |
Mn 55 |
| 5 |
Cu 64 |
Zn 65 |
? ?? |
? ?? |
As 75 |
Se 79 |
Br 80 |
| 6 |
Rb 85 |
Sr 87 |
Y 89 |
Zr 91 |
Nb 93 |
Mo 96 |
? ?? |
| 7 |
Ag 108 |
Cd 112 |
In 115 |
Sn 119 |
Sb 122 |
Te 128 |
I 127 |
Atomic weights of the elements are shown along with the symbols, to
illustrate that the elements have been placed in the table in order of
increasing atomic weight. Notice the gaps (highlighted in yellow)
that sometimes had to be left in the table. Consider row 5, for example.
Even though no elements with atomic weights between zinc (Zn) and arsenic
(As) were known at the time, Mendeleev did not place arsenic immediately
after zinc. This would have placed arsenic in the same column as
boron and aluminum, but arsenic is not chemically similar to these elements.
Based on its chemical properties, arsenic belongs in the same column as
nitrogen and phosphorous, so Mendeleev left two gaps in the table, and
placed arsenic where its chemical properties indicated it should go.
He reasoned that the gaps belonged to elements that had not been discovered
yet. Based on the regular way in which the properties change as one
goes through the table, he was able to predict the properties of the missing
elements. When the missing elements were discovered, it was found
that his predictions were generally quite accurate, which lead to widespread
acceptance of the table.
Another problem with Mendeleev's table -- more disturbing than the missing
elements (yellow cells) -- was that sometimes elements had to be deliberately
placed out of order to make the elements fall in the columns that were
consistent with their chemical properties (red cells). Notice in
row 7, that if listing the elements strictly on the basis of atomic weight,
we would be forced to put I in column VI and Te in column VII. But
this would put the elements in columns that do not match their observed
chemical properties. So Mendeleev temporarily violated his own periodic
law, and placed the elements in the order that placed them in the proper
columns, with regard to their chemical properties. At the time, Mendeleev
assumed that the atomic weights of tellurium and iodine had been incorrectly
measured (they are close, after all) and he believed that a "correct" measurement
would reveal that the atomic of tellurium was less than that of iodine.
But that was not the case. We know today that the elements must be
listed in order of increasing atomic number, not increasing atomic weight.
When this is done, discrepancies like that seen with tellurium and iodine
disappear, and all elements naturally fall into their rightful place in
the periodic table.
The arrangement of elements in the periodic table is based on the chemical
properties of the elements. The chemical properties, in turn, are
determined mainly by the number of electrons in the valence shell.
One reason we can be so confident in our modern understanding of the electronic
structure of the atom is that it explains the periodic table so well.
The next several pictures show Bohr model drawings of atoms, and an outline
of the modern periodic table, with the element's position highlighted.
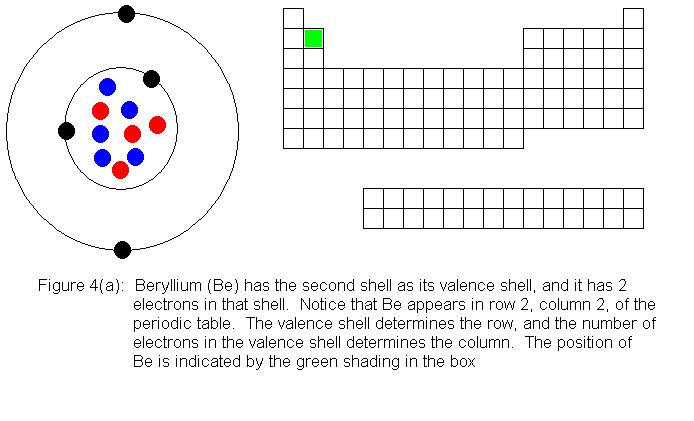
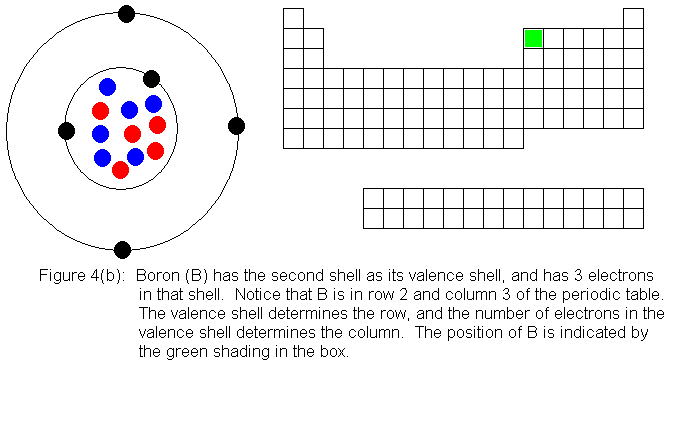
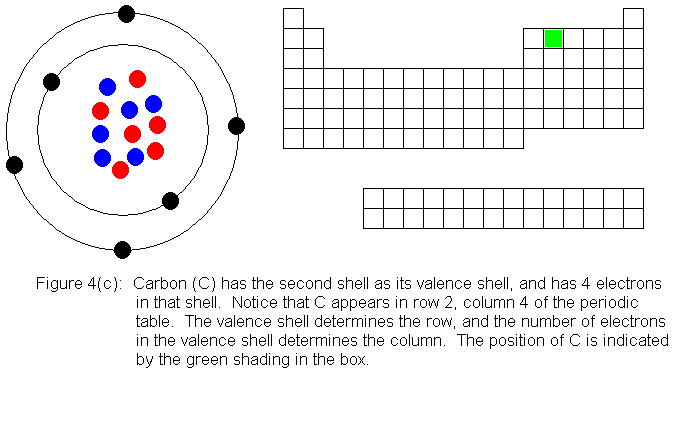
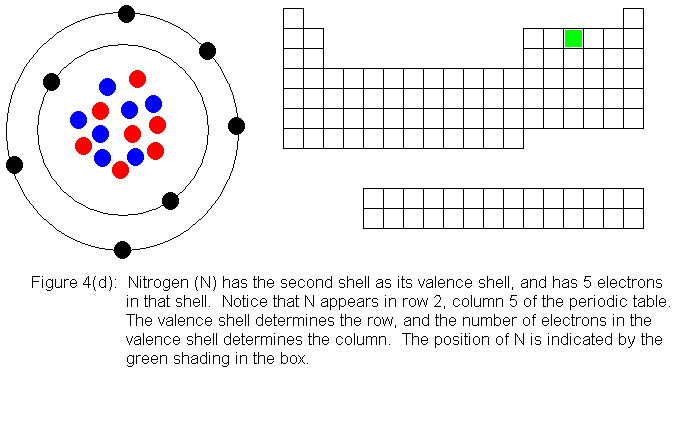
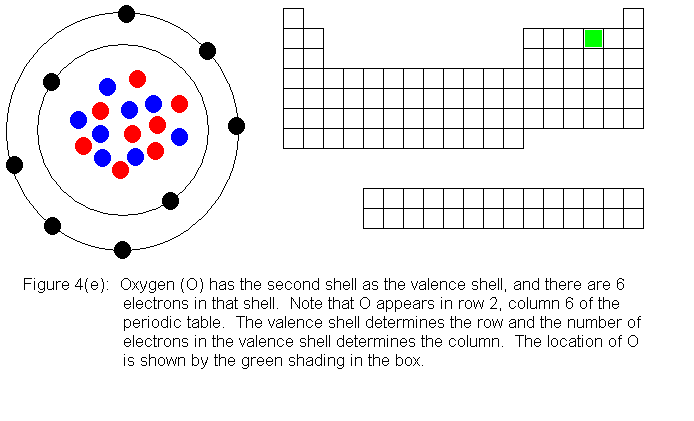
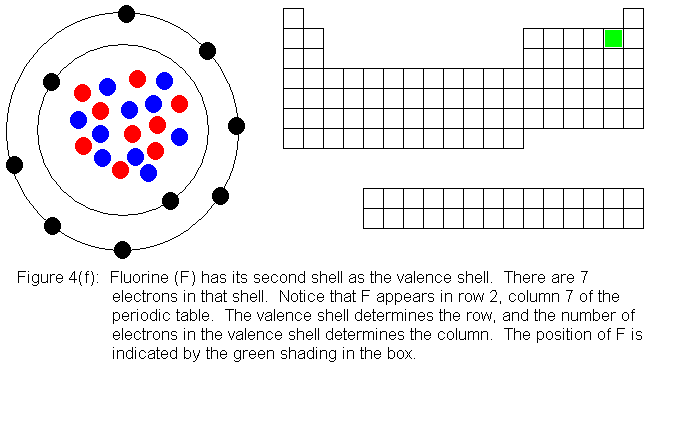
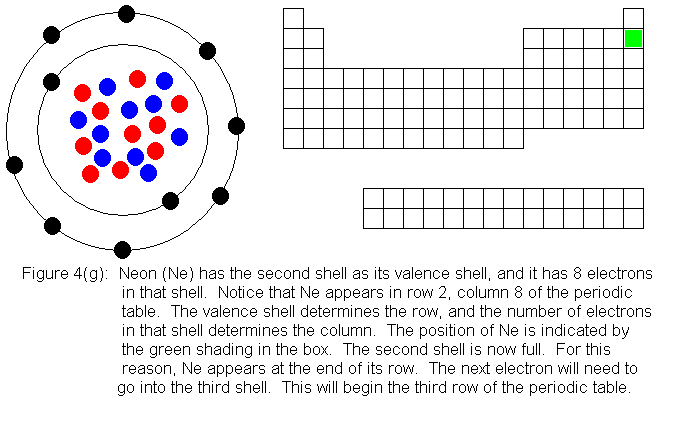
In the above series of pictures, we see that as electrons are added
to the atom's valence shell, the element's position moves to the right
in the periodic table. When an atom's shell becomes full, the atom's
positon has reached the end of the row. Not counting the transition
elements (the 10 shorter columns in the middle of the table) there are
8 columns. The elements in the first 2 columns and the last 6 columns
are called main group elements, or representative elements. These
are elements in which electrons are being added to the valence shell.
With the exception of the first shell, valence shells can hold 8 electrons,
and are considered full when they have this number. This explains
why the main group elements are 8 columns wide.
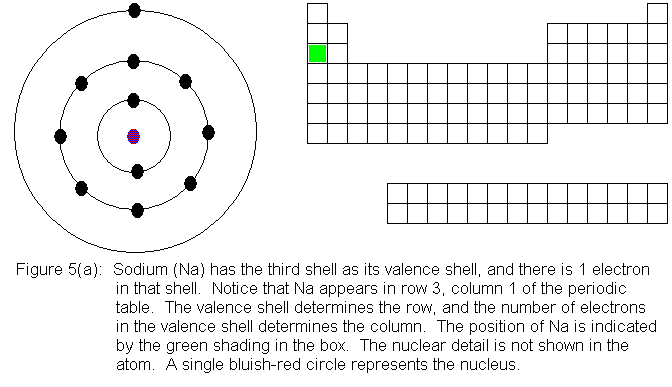
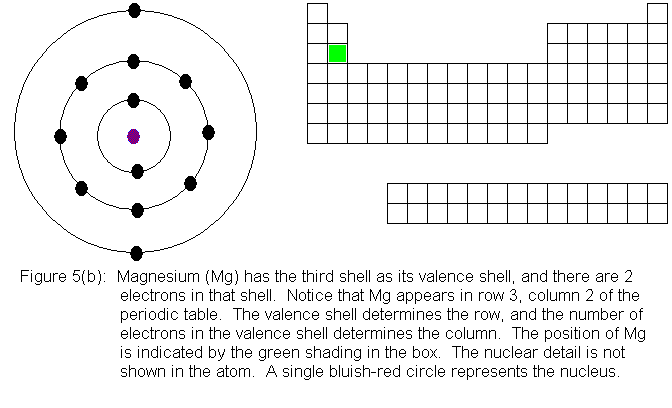
When we begin adding electrons to the third shell, we encounter, for
the first time, a shell for which the absolute maximum electron capacity
is larger than the valence shell capacity. While the n=3 shell is
the valence shell, it can hold only 8 electrons, but when a pair of electrons
is present in the n=4 shell, the n=3 shell gains the ability to hold an
additional 10 electrons, reaching its true limit of 18 electrons total.
The third row in the periodic table fills out in the same manner as the
second row.
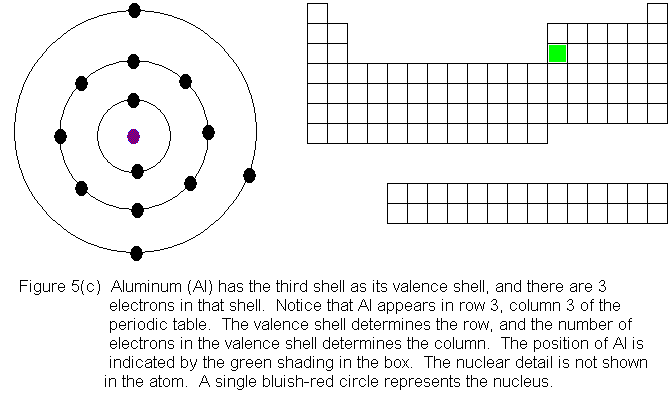
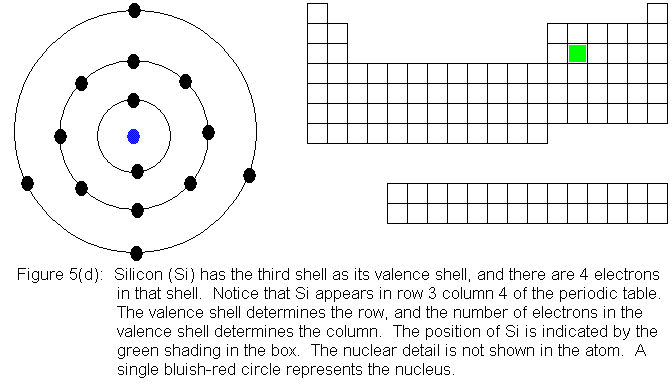
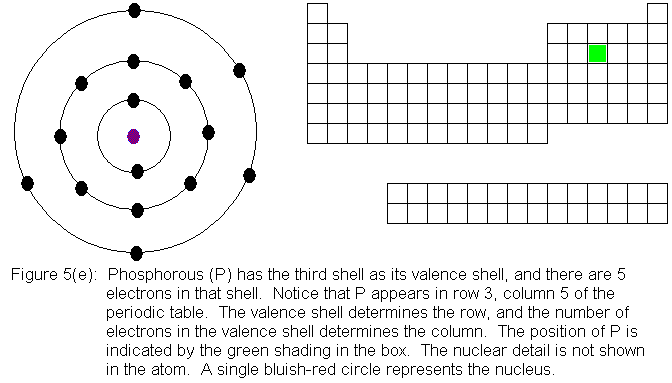
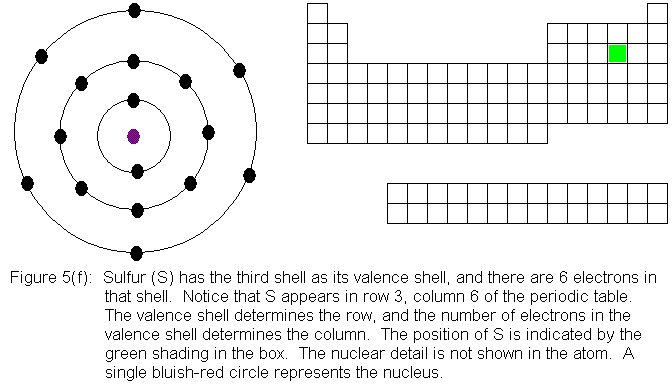
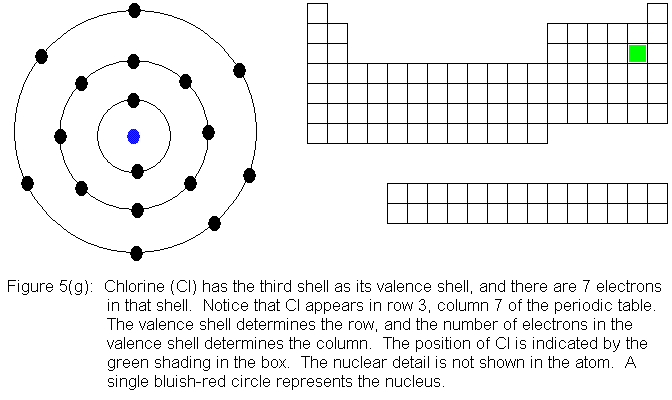
In the above series of pictures, electrons were added one by one to
the third shell, as we moved across the third row of the periodic table.
When we reached 8 electrons in this shell, the shell was "full" because
as a valence shell, that's all it holds. The next electron we place
in the atom will have to go into the fourth shell, so once the next electron
is added, the fourth shell -- not the third shell -- will be the valence
shell. For this reason, you might think that immediately after the
first electron enters the fourth shell, the third shell would continue
filling. However, it turns out that electrons tend to be dealt with
in pairs, so we will actually need TWO electrons in the fourth shell before
the third shell begins filling again.
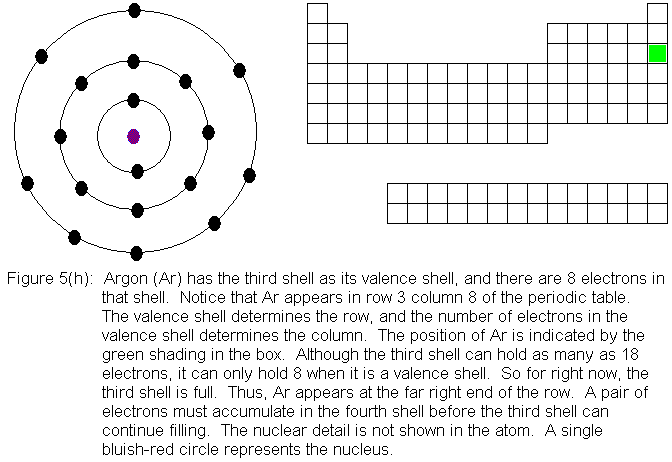
Now that the fourth shell has a pair of electrons, the third shell
"recognizes" its true capacity of 18 electrons. Since it only has
8 electrons at this point, it begins filling again. It can take on
10 more electrons to reach its true capacity. Notice that we are
about to enter a section of 10 shorter columns in the periodic table.
This part of the table did not exist in rows 1, 2, and 3. Notice
that we had a huge gap between columns 2 and 3 in those rows. This
gap is filled in rows 4 and below with the transition elements.
These are elements in which electrons are being added to the shell immediately
behind the valence shell, rather than to the valence shell itself.
Thus, for the transition elements in row 4, electrons are being added to
shell 3, and for the transition elements in row 5, electrons are being
added to shell 4, and so on.
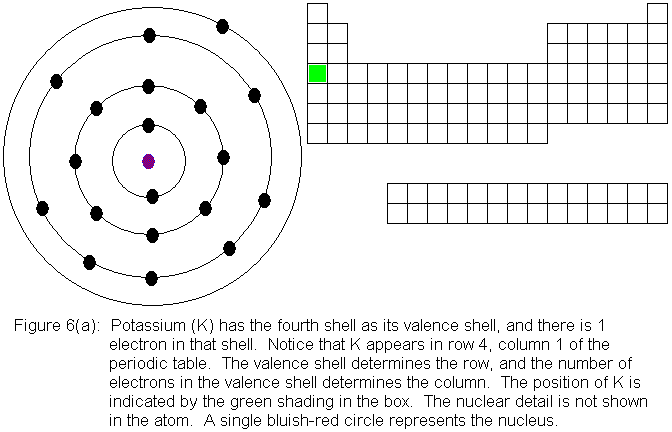
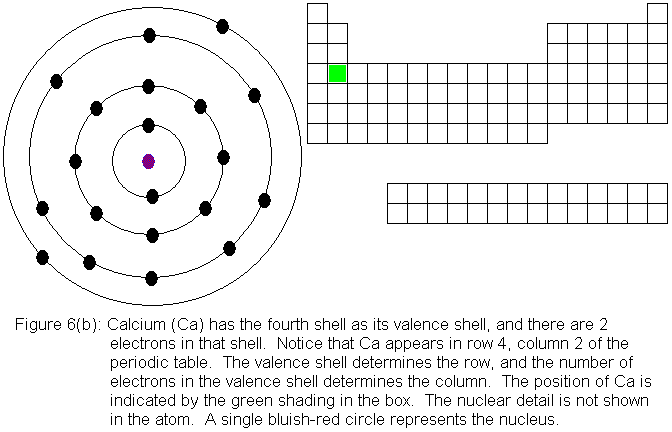
You might be wondering what happens to the numbering of columns at this
point. We had previously referred to the colum that contains the
element boron (B) as column 3. Now that we have to begin considering
the transition elements, does boron's column become column 13? While
we sometimes number all the columns sequentially in this manner, it is
often more useful to consider them separately and distinguish them
by letters. In our general chemistry courses here at Palo Alto College,
we most often designate the 8 columns we considered from early on with
the letter A, and the group of 10 that we have just now begun to consider
with the letter B. Unfortunately, the use of the letters A and B
is not universally recognized. However, we can eliminate confusion
by referring to the elements in the "A-groups" as main group elements,
also called representative elements.
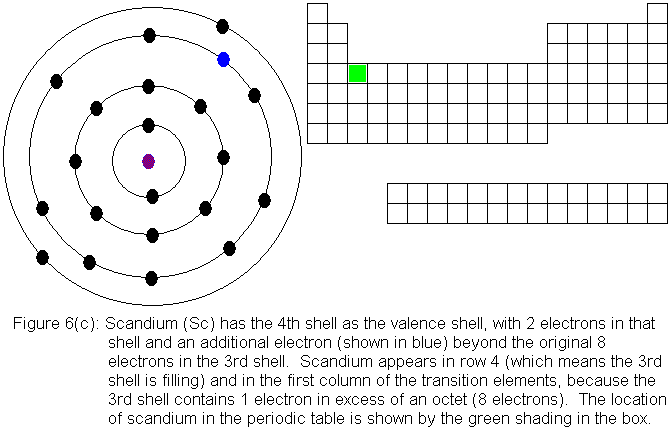
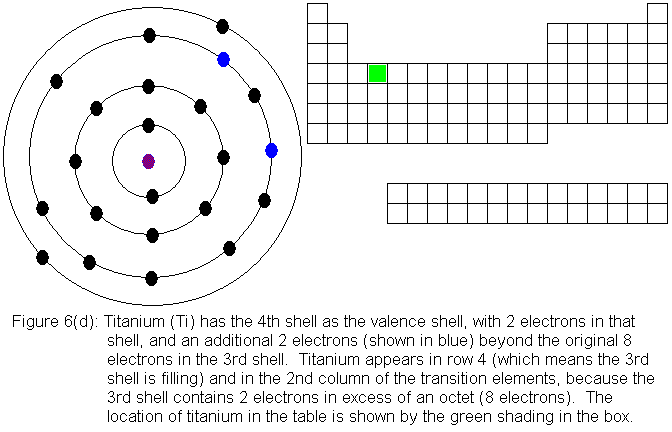
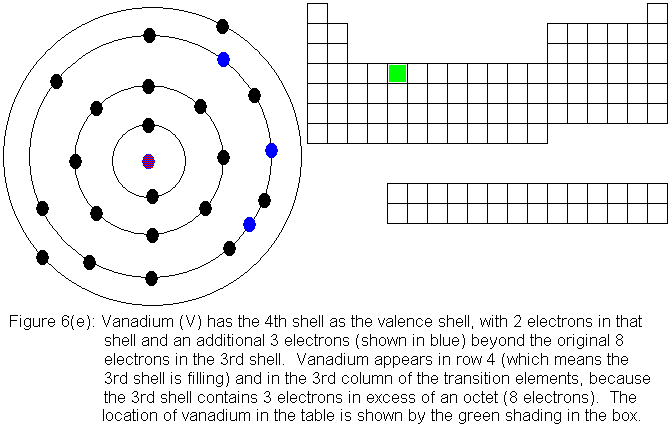
So far, the electronic structure of atoms has followed a pattern without
exception. It would be nice if this were the case with all the remaining
elements, but unfortunately, there are a number of elements that break
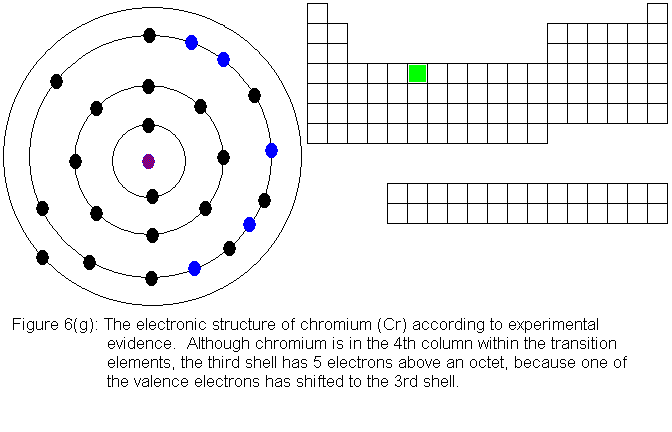
the pattern, and chromium is the first of several exceptions. In
the transition elements so far considered, each new element in sequence
adds an additional electron to the third shell, and the fourth shell (the
valence shell for the series of elements we are now considering) is left
alone. In chromium, we can think of the situation this way: when
we add a fourth electron above the octet in the third shell, one of the
valence electrons drops from the fourth shell to the third shell.
We end up with 5 electrons above an octet (rather than 4) in the third
shell, and only 1 valence electron. The predicted and experimental
electronic structures are shown in Figures 6(f) and 6(g) respectively.
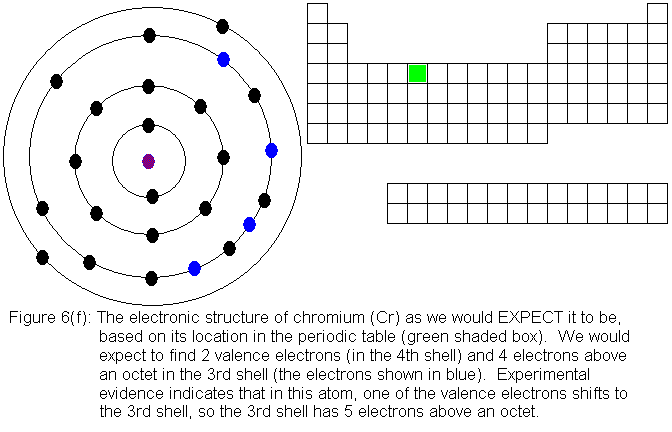
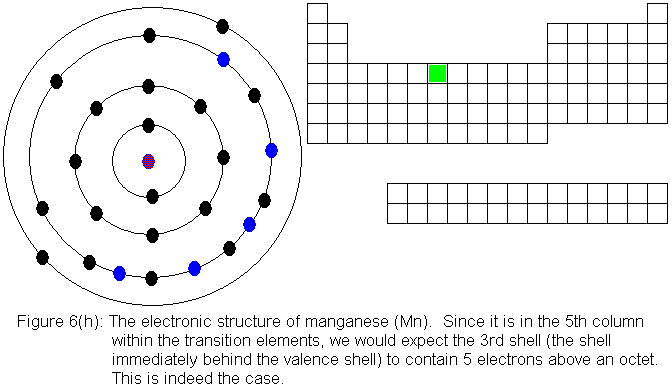
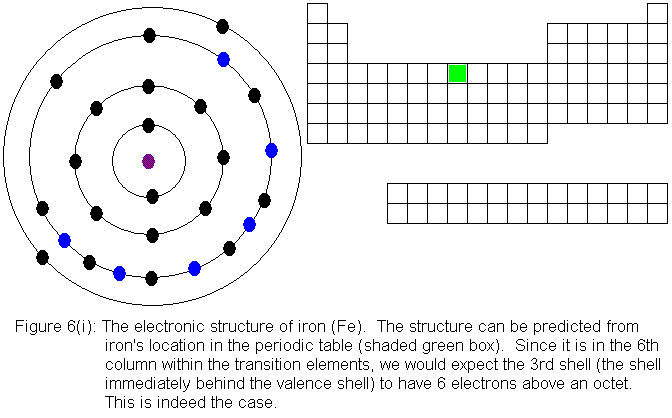
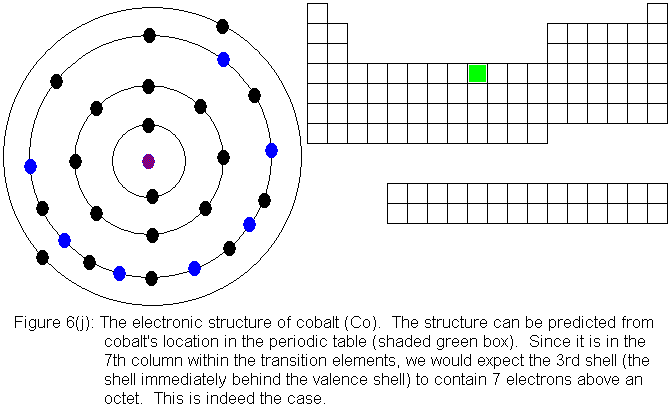
For the remainder of the transition elements in the fourth row, all
except copper follow the expected pattern. Copper deviates in the
same manner as chromium -- that is, a fourth shell (valence shell) electron
shifts to the third shell. The electronic structures are shown in
the following figures. For copper, only the actual electronic structure
is shown, but it is noted in the figure that the structure deviates from
that predicted by location in the periodic table.
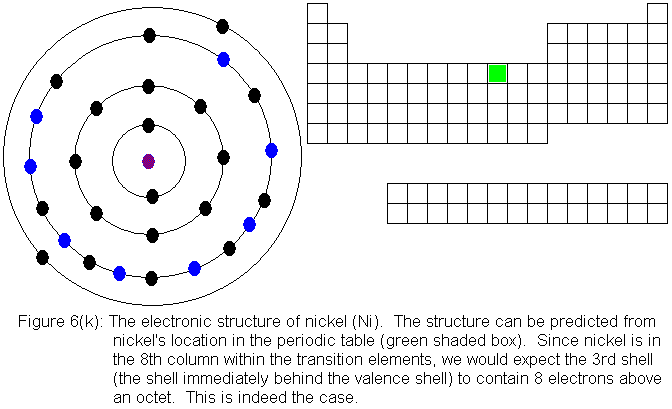
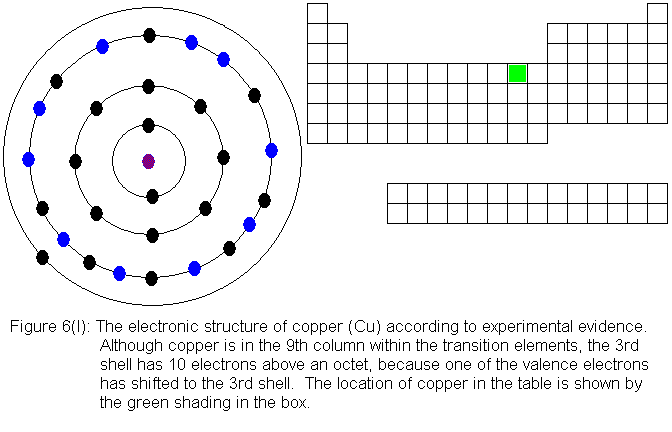
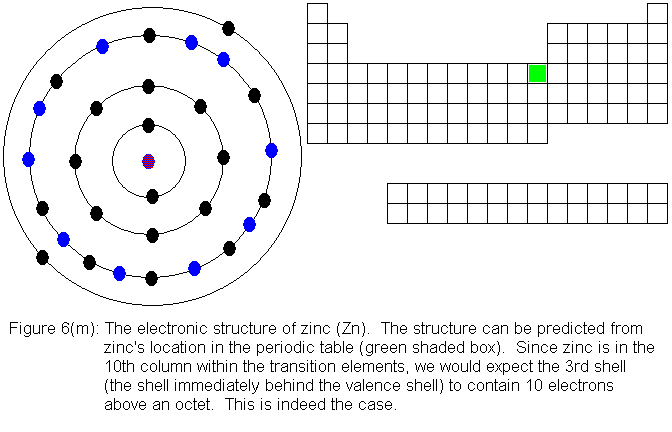
With the element zinc (Figure 6m) the third shell has been filled to
capacity. As we continue to add electrons to the atom, the fourth
shell (valence shell) begins filling again. It currently has 2 electrons,
and will fill to 8 in the manner expected. Upon receiving its 8th
electron, it will be temporarily "full" (because it is a valence shell)
and the 5th shell will start to fill. The next series of figures
shows the filling of the fourth shell to 8 electrons.
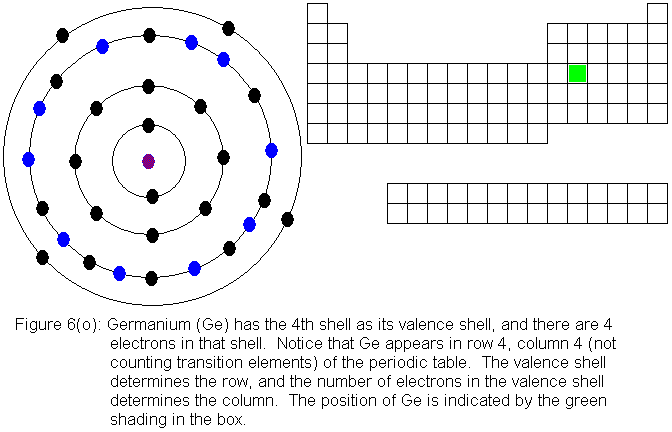
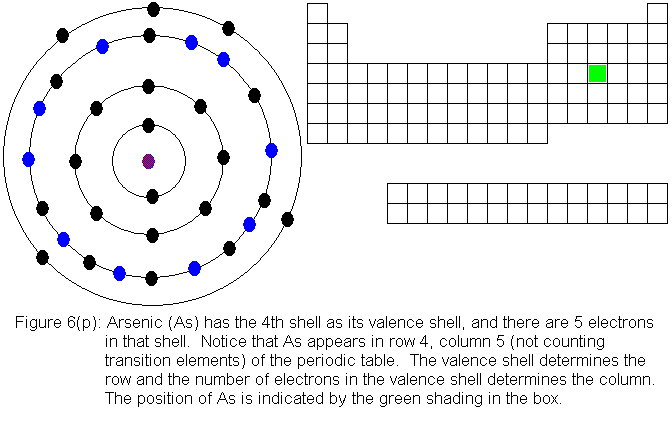
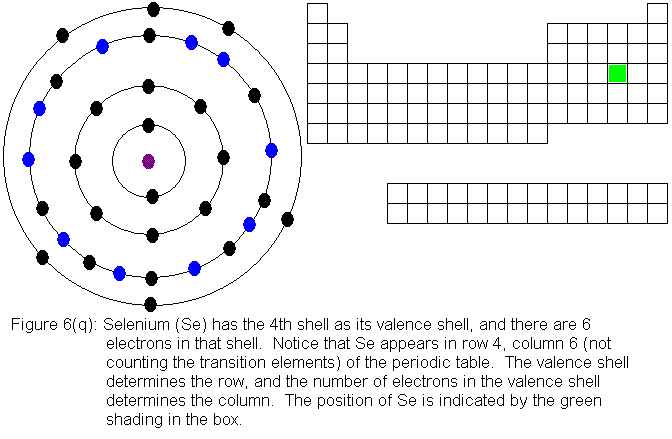
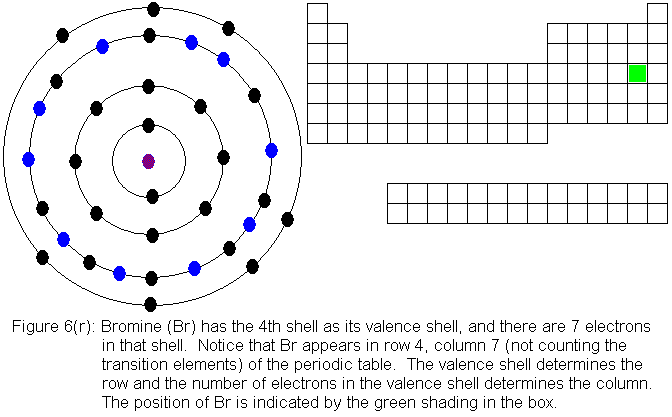
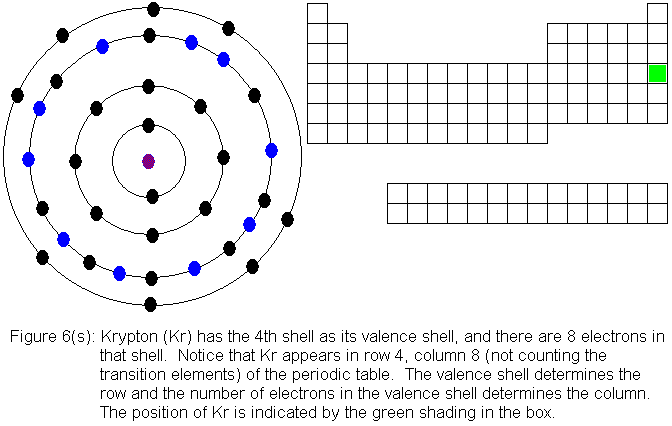
The fourth shell, since it is the valence shell, is now "full" with
8 electrons. Its true capacity is 32 electrons, so it can hold 24
more electrons than it now holds. However, the fifth shell must take
on a pair of electrons before the fourth shell "realizes" that it can hold
more electrons. After the fifth shell takes on a pair of electrons,
the fourth shell will take on 10 more electrons as we proceed across the
block of transition elements, which we have seen, is 10 elements wide.
But that takes care of only 10 electrons of the 24 additional electrons
we said this shell could hold. There are still 14 more electrons
that the fourth shell can hold. It will acquire those electrons as
we go across the block of inner-transition elements. Notice
the two long rows of elements below the main body of the periodic table.
If you count the number of columns, you will find that there are 14 --
accounting for the remaining electron capacity of the fourth shell.
Earlier, we saw that in the transition elements, electrons are being added
to the shell immediately behind the valence shell, rather than to the valence
shell itself. The inner-transition elements are those elements in
which electrons are being added to a shell that is TWO shells behind the
valence shell. Thus, the final 14 electrons will not be added to
the fourth shell until the sixth shell takes on a pair of electrons.
We will see this as we proceed through the rest of the periodic table.
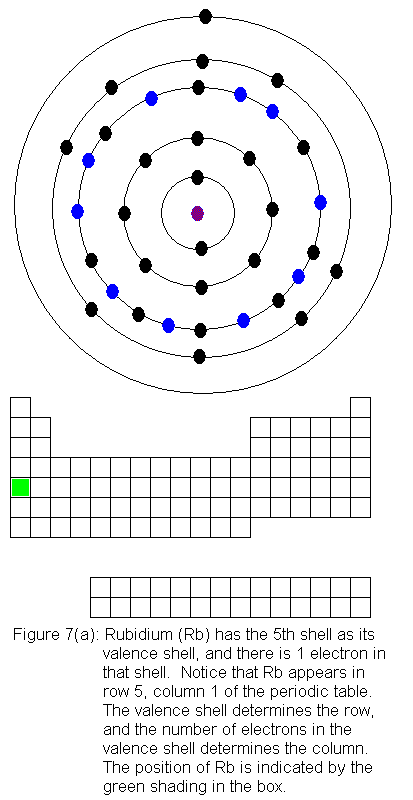
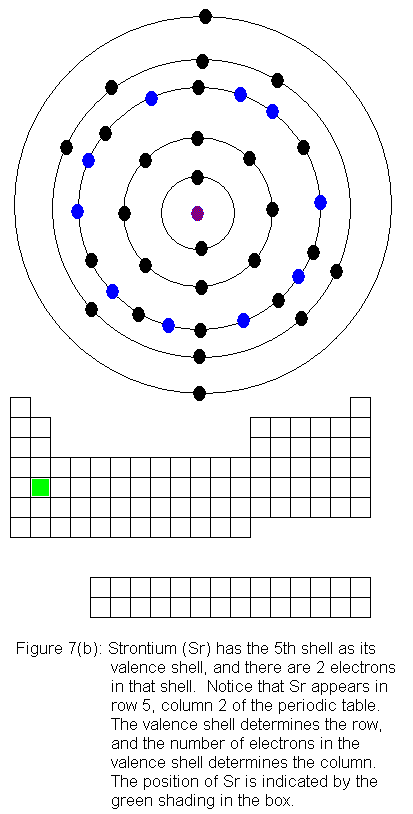
The fifth shell now has a pair of electrons, and the fourth shell begins
filling again. This occurs as we go across the transition elements
in the fifth row of the table (remember, in the transition elements, electrons
are being added to the shell behind the valence shell). Six of the
10 elements in this row have electron configurations that would not be
predicted from their locations in the periodic table. As before,
the discrepancies result from valence shell electrons shifting to the shell
immediately behind the valence shell. In this case, it means fifth
shell electrons shifting to the fourth shell. In what follows, I
present the actual electron configurations as indicated by experimental
evidence and note in the caption that the configuration is not the expected
one.
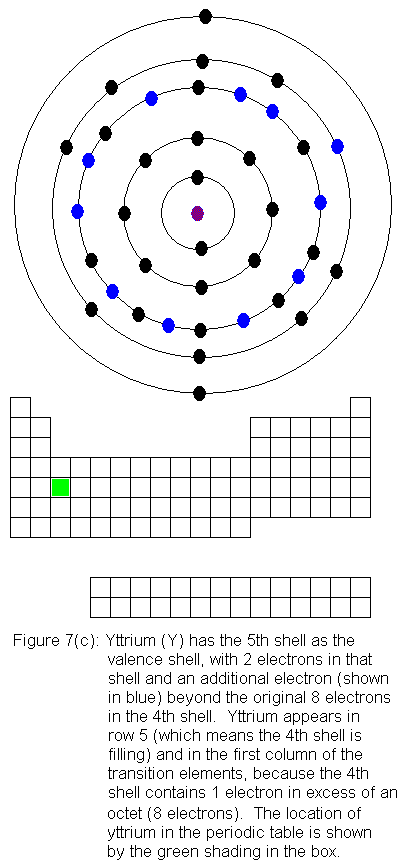
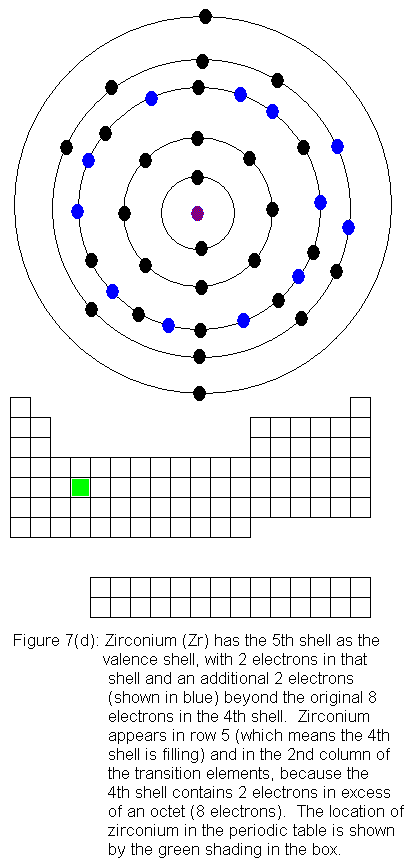
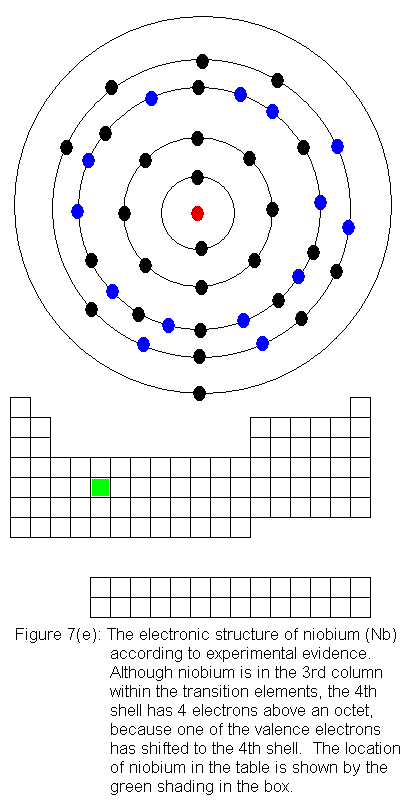
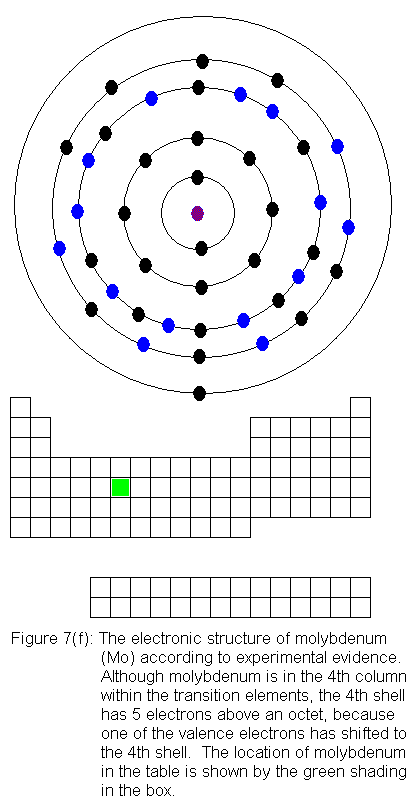
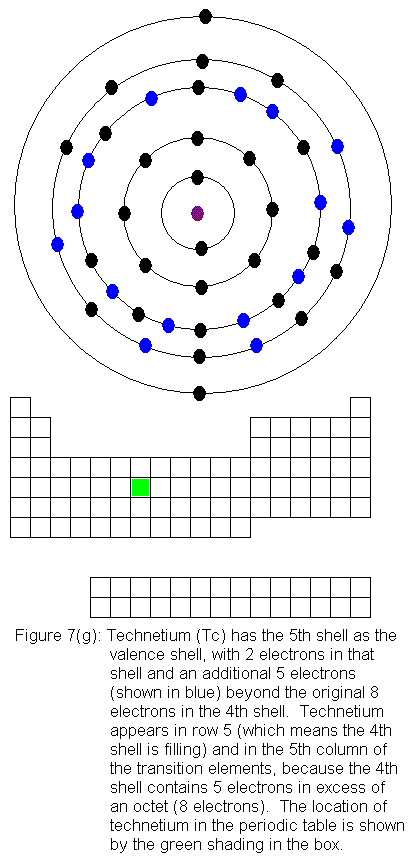
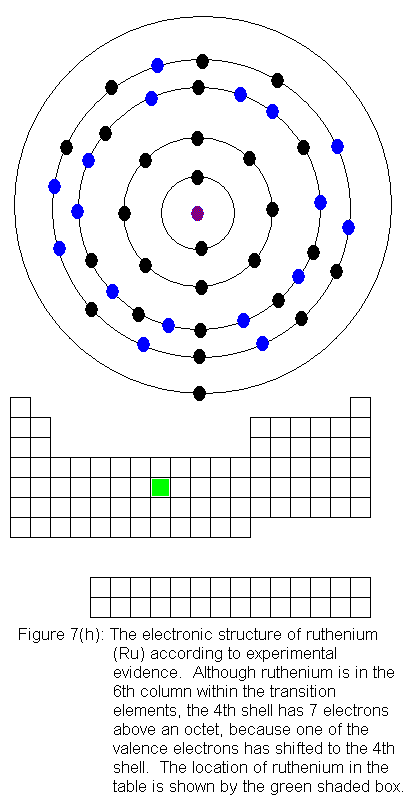
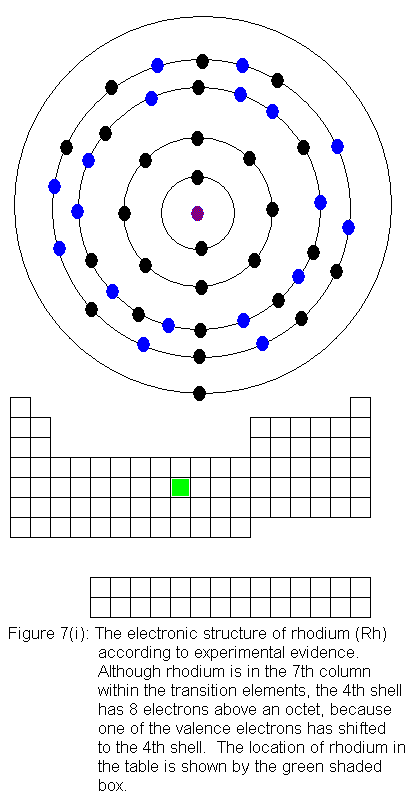
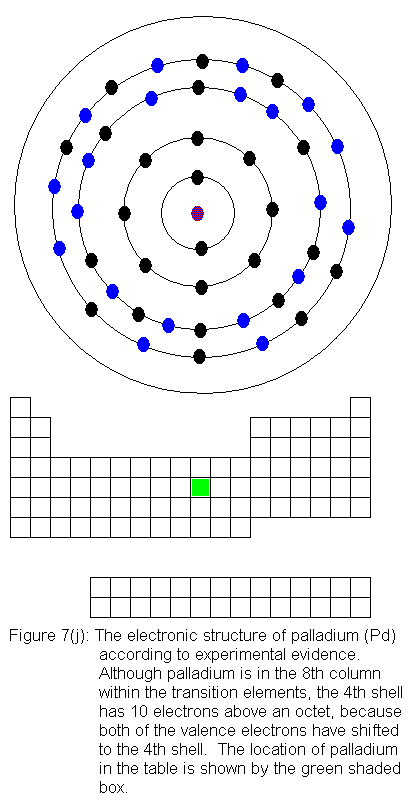
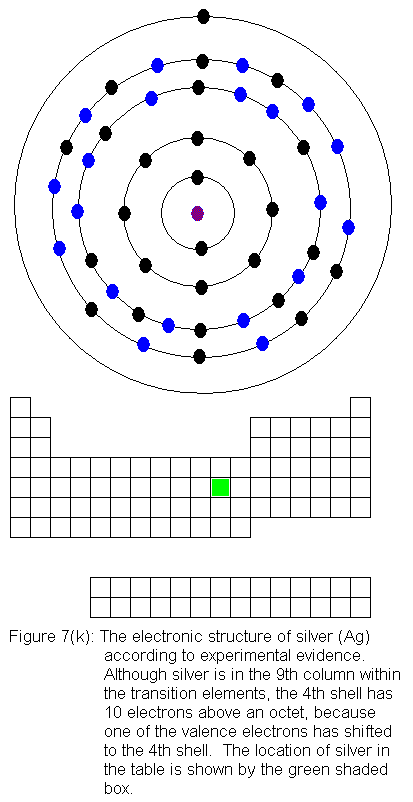
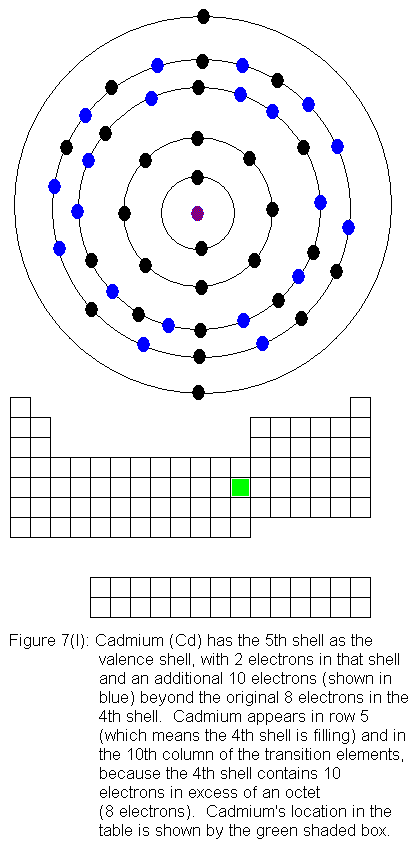
The fourth shell now contains 18 electrons. It can hold 32 electrons,
which leaves room for 14 more, but it will not acquire those until the
sixth shell takes on a pair of electrons. The 14 remaining electrons
are "inner-transition electrons", which means they are added to a shell
two shells behind the valence shell. Before the sixth shell can begin
taking electrons, we must fill the fifth shell up to an octet, which makes
it temporarily full (since it is currently a valence shell). The
next set of pictures shows the Bohr model drawings and periodic table locations
for indium (In) through xenon (Xe).
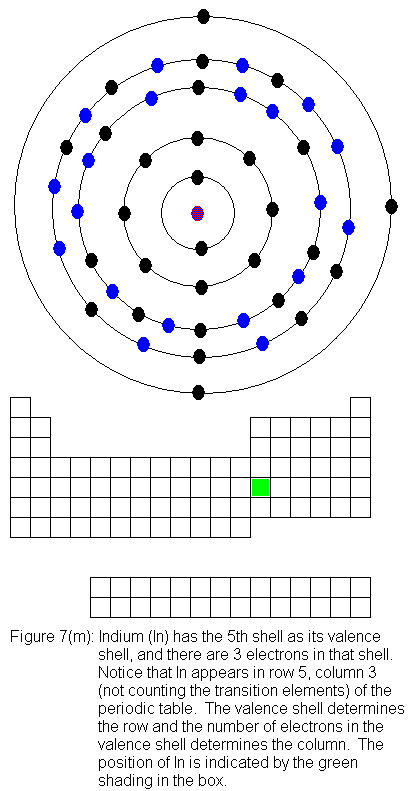
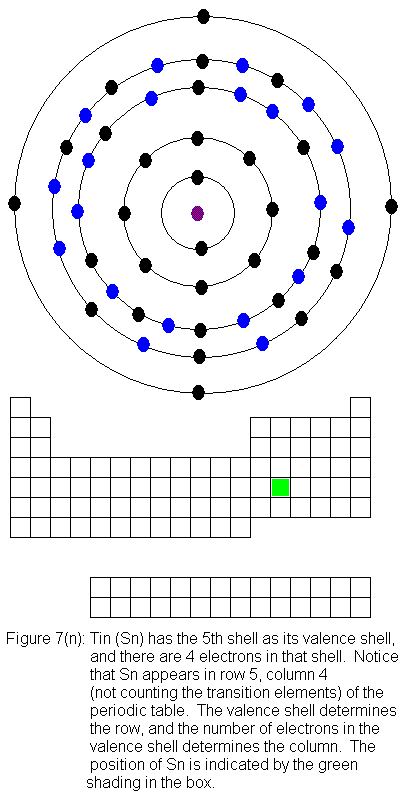
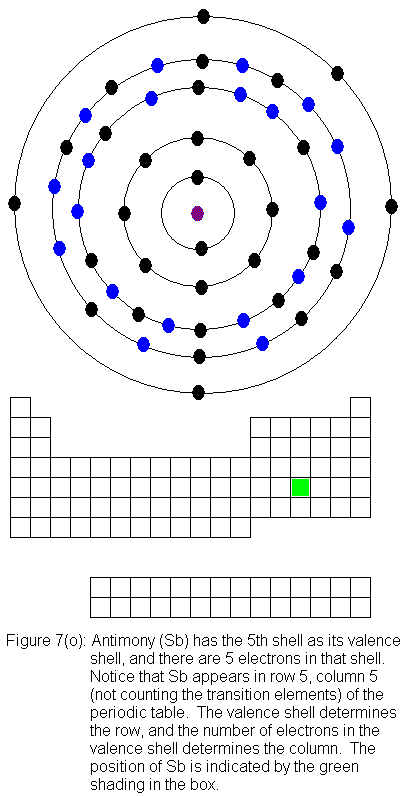
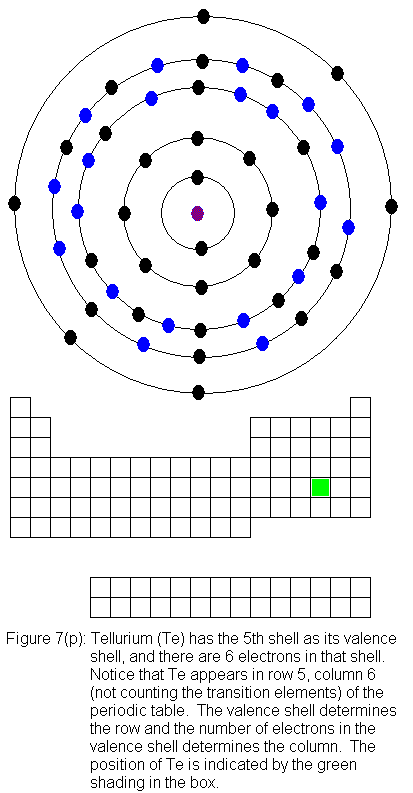
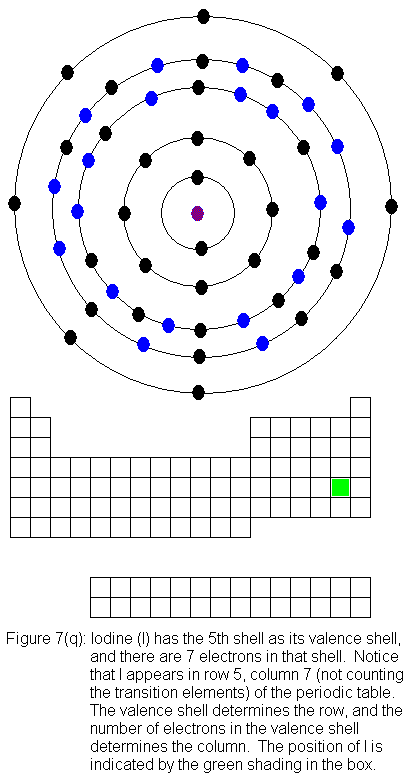
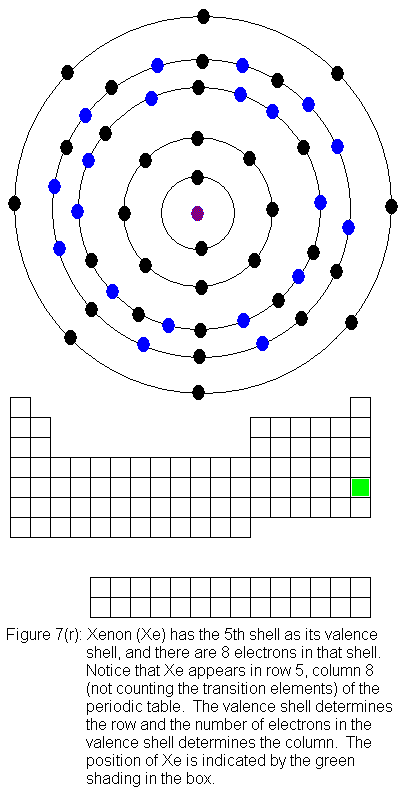
The fifth shell (because it is still a valence shell) is now temporarily
full with 8 electrons. The next 2 electrons (because they tend to
come in pairs) will enter the sixth shell. After that, the general
feature is that the fourth shell will fill, taking the remaining 14 electrons
needed to fill it to capacity, followed by filling the fifth shell from
its current 8 electrons to 18 electrons. However, there will be a
few exceptions to this general pattern. As we consider the first
row of inner-transition elements (the lanthanide series, so called because
it follows the element lanthanum), we will see that the exceptions to the
pattern arise from a shift of fourth shell electrons to the fifth shell.
In other words, the fifth shell does not always "wait" for the fourth shell
to fill before taking on additional electrons. There will be some
configurations in which an electron has been removed from the fourth shell
and added to the fifth shell. These exceptions (like the pattern
exceptions we have seen previously) arise because the electron energy levels
in question are extremely close together. As a result, subtle effects
can make a difference in which energy level is higher. The unifying
principle in all of this is that electrons will always fill the lower energy
states before the higher ones. The apparent random disruption of
what would otherwise be a very nice predictable patters is simply nature's
way of ensuring that the set of electrons has the minimum possible energy.
The next set of pictures will take us through the lanthanide series
of inner transtion elements.






















































