| IONIC | MOLECULAR |
|---|---|
| A compound composed of a metal and a non-metal | A compound composed of two non-metals |
| A compound composed of a metal and a polyatomic anion | A compound composed of a metalloid and a non-metal |
| A compound composed of a polyatomic cation and a non-metal | A compound composed of hydrogen and a non-metal (can often be classified as binary acids) |
| A compound composed of a polyatomic cation and a polyatomic anion | A compound composed of hydrogen and what would normally be considered a polyatomic anion (most compounds in this class are oxoacids) |
As you may alreday know, an atom contains smaller particles which we call subatomic particles. Three such particles that we will consider in this course are the proton, the neutron, and the electron. Each subatomic particle of a given kind is identical to any other subatomic particle of the same kind. For example, it is not possible to distinguish one electron from another.
As their name suggests, neutrons are electrically neutral -- they have no charge. Each proton carries a positive charge, and each electron carries a negative charge. The magnitude of the charges of the proton and the electron are equal. The only difference is that they have opposite signs. In SI units, electric charge is expressed in units called coulombs (C). The charge of the proton is +1.60 x 10-19 C and that of the electron is -1.60 x 10-19 C. Because we will always deal with electric charges in multiples of this amount (you can only have a whole number of protons and electrons) it is convenient to call an amount of electric charge (whether positive or negative) equal to 1.60 x 10-19 C "1 unit of charge". Therefore, we will normally work with this non-SI unit, rather than using the cumbersome coulomb units.
In atoms, the protons and neutrons are found in the atom's nuclues, while the electrons move around in the space outside the nucleus. The protons and neutrons have approximately equal masses, but are almost 2000 times heavier than the electron. As a result, almost all the mass of an atom is concentrated in its nucleus, but the electrons contribute most of the atom's volume.
The identity of each atom (that is, which chemical element it belongs to) is determined by the number of protons in the atom's nucleus. Thus any atom that has a single proton in its nucleus must be a hydrogen atom and likewise any atom that is known to be a hydrogen atom must have a single proton in its nuclues. Similar statements can be made for the other elements, except that the number of protons must be changed to what is appropriate for that particular element. The number of protons in the nucleus is referred to as the atomic number. Thus we can say the atomic number of hydrogen is 1.
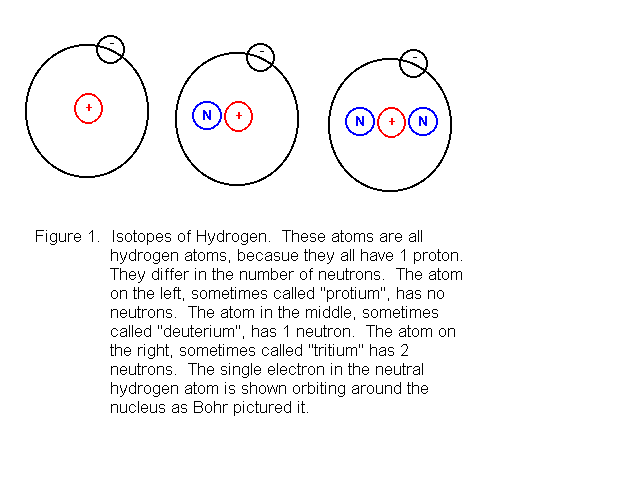
Originally, chemists thought that all atoms of the same chemical element were identical. In those days, they pictured atoms as solid spheres which were indistinguishible from others of their own kind but different from atoms of other elements. Today, however, we know that even atoms of the same chemical element can be different from each other. This difference arises because even though two atoms of the same element must have the same number of protons, they can have a different number of neutrons. Two or more atoms that are related in this way are said to be isotopes of each other. That is, isotopes are two or more atoms that have the same number of protons but a different number of neutrons. Three isotopes of hydrogen are known. Bohr model drawings of these atoms are shown in Figure 1.

In the Bohr model, electrons orbit around the nuclues, similar to the way planets in our solar system orbit around the sun. We now know that the motion of electrons in atoms is far more complicated than that described by Bohr's model. Nevertheless, it is still sometimes useful to picture atoms as shown in Figure 1. We can do this when we want to explain the arrangement of the periodic table in a simple way, and when we want to explain why particular atoms tend to take on one certain electric charge but no other charges. When we want to consider the electronic structure of the atom in more depth, or when the motions of the electrons are of considerable importance, we must abandon the Bohr model and use quantum mechanics instead.
The drawing in Figure 1 is sufficient here, because we only want to show what isotopes are, and that the atoms are electrically neutral. We can see the electrical neutrality by noting that in each atom shown in the figure, there is 1 proton, contributing 1 unit of positive charge, and 1 electron, contributing 1 unit of negative chagre. Since there are equal amounts of positive and negative charge, the charges cancel out, leaving an electrically neutral atom.
Except when we deal with nuclear chemistry, atoms do not gain or lose protons and neutrons. However, the gain or loss of electrons is a frequent occurance in chemistry. When electrons are gained or lost, the atom will no longer have an equal number of protons and electrons. As a result, the atom will carry an electric charge. We call an atom having an electric charge an ion. If an atom becomes an ion having a positive charge we call it a cation. If an atom becomes an ion having a negative charge we call it an anion.
Later, we will use the Bohr model to explain why atoms prefer some electric charges over others, and we will even delve into some elementary quantum mechanics. For now, however, I will teach you how to use the periodic table to predict the electric charge for the ions of several elements. For the purpose of writing names and formulas of compounds, we really don't need to know the underlying reasons why atoms take on particular charges, just as long as we know what those charges are.
To use the periodic table to predict charges of ions, we need to first know something about its structure. The periodic table can be divided into 3 sections: main group elements (also called representative elements), transition elements, and inner-transition elements. The location of these elements in the periodic table is shown in Figure 2.
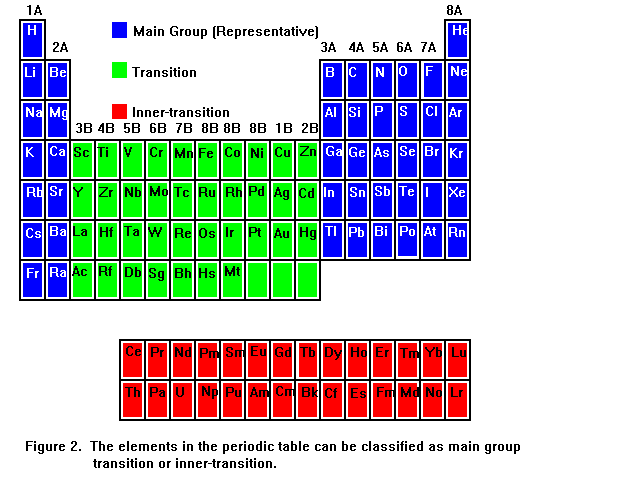
As you can see, the main group elements, also called representative elements, are the elements in the first 2 and last 6 columns in the main body of the table. These elements are shown in blue boxes with the symbols being given in white letters. The main group elements will be the easiest for us to deal with, because most of them only form ions of a single charge, and it will be clear from the location of the element in the table what the charge is. We will return to this point very soon.
The transition elements are those elements that appear in the 10 columns that split up the main group elements. They are shown in the figure in green boxes, with the element symbols being given in black letters. The transition elements are not quite as easy to deal with, because they typically form two different charges of ion. It will be necessary to take this into account when writing names and formulas of compounds containing transtion elements.
The inner-transition elements are shown below the main body of the table as red boxes in which the element symbols are given as black letters. We will seldom deal with them in any detailed way in this course.
Looking at the periodic table in Figure 2, you can see that the columns belonging to the main group elements are numbered 1 through 8 from left to right, and have a letter A at the top. The transition elements are numbered 3 to 8 from left to right, with 3 different columns being assigned the number 8, and the last 2 columns are then numbered 1 and 2. These columns all have the letter B above them. This is the system of lettering that is used in our Hill and Petrucci text, and it suits our purposes for using the periodic table to help us determine chemical formulas, but we must bear in mind that this notation is not universally used. Within the context of this course, we could say "A group element" to refer to a main group element and "B group element" to refer to a transition element, but this might cause confusion for someone outside our course, who is using a periodic table labeled differently. Some periodic tables switch the roles of the letters A and B, and perhaps even more confusing, some use the label A for the first 8 columns, then change from A to B in the middle of the transition elements. However, the terms "main group element" and "transition element" have the same meaning for everyone.
As was mentioned earlier, the main group elements are particularly easy to deal with. In the first 3 columns (of the main group -- that is, first 3 BLUE columns) the atoms form ions having a positive charge equal to the group number. Thus, when the sodium (Na) atom forms an ion, it will have a charge of +1 and we write it Na+. Similarly, potassium will form K+, Rubidium will form Rb+, and so on. Only the +1 charge is encountered for elements in this column. We would never encounter sodium with a +2 charge (Na2+) for example. Thus the charge is easily predictable from the loaction of the elements in the periodic table.
In the same way, we would predict Mg2+, Ca2+, and Sr2+ for the ions of magnesium, calcium, and strontium. Again, ions having charges other than that indicated by the group number (+2) are not seen for the elements in this column.
In group 3A, we expect ions having a charge of +3. Actually, the element boron (B) tends to form molecular rather than ionic compounds, so boron ions will not be encountered. This is because the boron atom, being the smallest atom in group 3A, would have a very high charge density if it were to become a B3+ ion. High charge densities are energetically unfavorable, so very small atoms tend not to take on high charges. For the same reason, looking back at group 2A, we find the beryllium tends to form molecular compounds, so we don't often see the Be2+ ion. The Li+ ion is known, because the charge is now low enough that the charge density is more acceptable. Still, we will seldom deal with lithium compounds in this course. If we do, however, we would predict Li+ for the ion.
A compliation occurs when when we consider the element thallium (Tl) at the bottom of group 3A. We would predict a charge of +3, writing the ion as Tl3+. These ions do indeed exist, but what one woud not have predicted based on the notes presented so far, is that thallium also forms the ion Tl+. Here for the first time, we see the phenomenon of multiple charges. This is the rule rather than the exception among the transition elements, but fortunately, it happens only rarely among the main group elements. When it comes to multiple charges, the only main group elements we need concern ourselves with are tin and lead, the last 2 elements at the bottom of group 4A. Tin forms Sn2+ and Sn4+, and lead forms Pb2+ and Pb4+. As in the case of thallium, we see that one of the two charges is the one we would have predicted based on the group number. Although the behavior of thallium has been noted here, it will not appear in any of the problems you will work in this course.
In group 4A, the elements carbon (C), silicon (Si) and Germanium (Ge) tend to form molecular compounds, and therefore not appear as monatomic ions. A charge of +4 is quite large as charges go, so the atom has to be quite large, before the charge density becomes acceptable.
Starting in group 5A, the atoms begin to take on negative charges rather than positive ones. Notice that this is also where we begin to encounter non-metallic rather than metallic elements. See the periodic table inside the front cover of your Hill and Petrucci textbook. It is color coded to let you see which elements are metals and which are non-metals. We will see that metals form postive ions (cations) and non-metals form negative ions (anions). Starting in group 5A, then, we usually encounter negative ions, and we can find the charge by subtracting 8 from the group number.
In group 5A, we have 5 - 8 = -3, so we expect N3- for nitrogen and P3- for phosphorous. This pattern is broken with bismuth at the bottom of the column because it is a metal. Remember that metals form positive ions. Bismuth forms Bi3+ ions.
In group 6A we have 6 - 8 = -2, so we expect O2- for oxygen and S2- for sulfur, the two elements we usually deal with in this column.
In group 7A we have 7 - 8 = -1 so we expect F-, Cl-, Br-, and I-. We will not deal with astitine (At).
In group 8A we have 8 - 8 = 0, so the elements in group 8A are not expected to form ions. These elements are chemically unreactive. They are called the noble gases, for that reason.
An ionic compound forms when atoms of one element lose electrons and those electrons are then gained by atoms of another element. The atoms losing electrons becomes positive ions, and the atoms gaining electrons become negative ions. The oppositely charged ions thus formed are then held together by electrostatic attraction (remember, opposite charges attract). This electrostatic attraction is called an ionic bond.
It is important to note that in the case of an ionic compound, there is no discrete particle that we can call a molecule. A molecule is a group of two or more atoms that are joined together by sharing electrons. The sharing of electrons is called a covalent bond. In an ionic compound, some atoms have lost electrons, and other atoms have gained electrons. Thus, there has been a transfer of electrons between atoms.
A consequence of the above is that chemical formulas of ionic compounds can only be written in lowest terms. In a crystal of table salt (sodium chloride) for example, there are an equal number of Na+ ions and Cl- ions. For every Na+ ion, there is a Cl- ion. However, it is not possible to pick out a particular Cl- ion as "belonging to" a particular Na+ ion. This is because the atoms are not joined together through a sharing of electrons. We write the formula of sodium chloride as NaCl to reflect the fact that there is a one-to-one ratio of Na+ ions to Cl- ions. It would be misleading to write the formula as Na2Cl2. Although it expresses the same ratio, the fact that it is not in lowest terms would imply that we are dealing with a molecule that contains 2 Na atoms and 2 Cl atoms, all covalently bonded together. Where molecules are involved, there is nothing wrong with having formulas with the subscrips not in lowest terms. We can write a molecular formula, that specifies the exact number of each kind of atom in the molecule. But ionic compounds do not exist as molecules, and it does not make sense to write such formulas in anything other than lowest terms.
Another requriement of ionic formulas is that they must be electrically neutral -- there must be equal amounts of positive and negative charge. We see this requirement met in the example above where sodium chloride has an equal number of Na+ ions and Cl- ions. The total charge will be zero, because every positive charge is cancelled out by a negative charge.
But not all ionic formulas are in a one-to-one ratio. Let's look at an example. Consider the compound magnesium chloirde. This compound would consist of magnesium ions and chloride ions. (When atoms become negative ions, the element name is changed to end in ide. Thus, chloride ions are the ions of chlorine.) From their locations in the periodic table, we expect magnesium ions to be Mg2+ (since Mg is in group 2A) and chloride ions to be Cl- (since Cl is in group 7A). Since each magnesium ion has twice as much positive charge as each chloride ion has negative charge, there must be twice as many chloride ions as magnesium ions in any sample of magnesium chloride. Therefore the correct formula is MgCl2. Notice that this formula is both neutral and in lowest terms (1 to 2 ratio). Proof of electrical neutrality is shown below:
1 x (+2) + 2 x (-1) = 0
Total
Total
Mg
Cl
charge
charge
The charges add up to zero, as required.
The foolproof mathematical procedure for obtaining the subscrips that
are both in lowest terms and generate an electrically neutral formula is
as follows:
Example 1: Predict the name and formula of the compound formed from the elements bromine and magnesium.
Solution:
Since bromine is a non-metal and magnesium is a metal, we expect the compound to be ionic. We name an ionic compound simply by naming the ions it contains. When doing this, we name the positive ion first and the negative ion second. Note that when an atom forms a negative ion, the name of the element is changed to end in ide. Thus from bromine, we will get bromide ions. For the positive ions, the original name of the element is not modified. Thus, from magnesium, we will get magnesium ions. We name the positive ion first and the negative ion second, so the name will be magnesium bromide.
To get the formula, we need to consider the ion charges. Magnesium is in group 2A of the periodic table, so is expected to form Mg2+. Bromine is in group 7A of the periodic table, so is expected to form Br-. The charges encountered (without regard to sign) are 2 and 1. The LCD of 2 and 1 is 2. The subscript on each ion is calculated by dividing the LCD by the ion charge.
For Mg2+ we have 2 / 2 = 1
For Br- we have 2 / 1 = 2
Therefore the formula of magnesium bromide is MgBr2. The subscript of 1 on Mg is understood and not written.
Example 2: Predict the name and formula of the compound formed from the elements aluminum and oxygen.
Solution:
Since aluminum is a metal and oxygen is a non-metal, we expect the compound to be ionic. When atoms become positive ions, the name of the element is not modified. Therefore, from the element aluminum, we will get aluminum ions. When atoms become negative ions, the name of the element is changed to end in ide. Thus, from oxygen, we get oxide ions. In naming the compound, we name the positive ion first and the negative ion second. Therefore, the name will be aluminum oxide.
To obtain the formula, we must consider the ion charges. Aluminum is in group 3A of the periodic table, so the expected ion is Al3+. Oxygen is in group 6A of the periodic table, so the expected ion charge is O2-. The charges encountered (without regard to sign) are 3 and 2. The LCD of 3 and 2 is 6. The subscript on each ion is calculated by dividing the LCD by the ion charge.
For Al3+ we have 6 / 3 = 2
For O2- we have 6 / 2 = 3
Therefore, the correct formula for aluminum oxide is Al2O3.
Example 3: Predict the name and formula of the compound formed from the elements calcium and nitrogen.
Solution:
Since calcium is a metal and nitrogen is a non-metal, the compound will be ionic. We name an ionic compound by naming the ions it contains, listing the positive ion first. Metals form positive ions, and the name of the element is not modified when a positive ion is formed. Thus from the element calcium, we will get calcium ions. Non-metals form negative ions, and the name of the element is changed to end in ide. Thus, from the element nitrogen, we will get nitride ions. Therefore, the name of this compound will be calcium nitride.
To get the formula, we need to consider the ion charges. Calcium is in group 2A of the periodic table, so is expected to form Ca2+ ions. Nitrogen is in group 5A of the periodic table and is expected to form N3- ions. The charges encountered (without regard to sign) are 2 and 3. The LCD of 2 and 3 is 6. The subscript of each ion is calculated by dividing the LCD by the charge of the ion.
For Ca2+ we have 6 / 2 = 3
For N3- we have 6 / 3 = 2
Therefore, the correct formula of calcium nitride is Ca3N2.
Example 4: Predict the name and formula of the compound formed from the elements aluminum and chlorine.
Solution:
Since aluminum is a metal and chlorine is a non-metal, the compound will be ionic. We name an ionic compound by naming the ions it contains, listing the positive ion first. Metal elements form positive ions, and the name of the element is not modified when forming the name of the ion. Non-metal elements form negative ions, and the name of the element is changed to end in ide when forming the name of the ion. Thus from the element aluminum, we get aluminum ions, and from the element chlorine, we get chloride ions. The name of the compound is therefore, aluminum chloride.
To get the formula, we need to consider the ion charges. Aluminum is in group 3A of the peroidic table, and therefore expected to form Al3+ ions. Chlorine is in group 7A of the periodic table, and therefore expected to form Cl- ions. The charges encountered (without regard to sign) are 3 and 1. The LCD of 3 and 1 is 3. The subscript on each ion is calculated by dividing the LCD by the charge of the ion.
For Al3+ we have 3 / 3 = 1
For Cl- we have 3 / 1 = 3
Therefore, the correct formula of aluminum chloride is AlCl3. The subscript of 1 on Al is understood and not written.
As the above examples show, it's not difficult to write chemical formulas for ionic compounds when the ion charges are readily known. There are a few ions, however, than can exist as two different charges of ion. For example, the element tin can exist in ionic form either as Sn2+ or Sn4+. This can lead to ambiguity when referring to the ions. If I say "sodium" ion, I do not need to specify that the charge is 1+, because sodium does not form ions of any other charge. Therefore, everyone understands that "sodium ion" refers to Na+. But when I say "tin ion", it is not clear whether I mean Sn2+ or Sn4+. There are two systems for dealing with this ambiguity.
Sn2+ is the tin(II) ion
Sn4+ is the tin(IV) ion
This is the preferred system because it explicitly gives the charge on the metal ion. Thus from the Stock name, you can immediately write the ion. For example, given the name chromium(III) ion, you know you are dealing with Cr3+. Most ions that exist as more than one charge have two known charges. Metals forming three or more different charges are rare, but they can easily be dealt with in the Stock system. For example:
Mn2+ is the manganese(II) ion
Mn3+ is the manganese(III) ion
Mn4+ is the manganese(IV) ion
The Stock system could easily handle any number of charges of the metal ion. You just include the appropriate Roman number in the parentheses. It is not necessary to specify the sign of the charge, because metals always form ions with positive charges. Thus in a Stock name, the Roman number is understood to indicate the magnitude of the positive charge.
Just as the term "tin ion" is ambiguous, so is the chemical name "tin chloride". Do I mean the compound containing Sn2+ ions or the compound containing Sn4+ ions? Since there are two different ion charges for tin, there can be two different tin chlorides. If we are dealing with tin(II) ions, then we deduce the formula as follows:
The ions are Sn2+ and Cl-.
The charges encountered are (without regard to sign) 2 and 1. The LCD of 2 and 1 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Sn2+ we have 2 / 2 = 1
For Cl- we have 2 / 1 = 2
Therefore the correct formula for the compound containing these ions is SnCl2. The name of this compound is tin(II) chloride because it contains tin(II) ions -- NOT because it has two chloride ions in the formula. Take note of this, because it is a misconception students sometimes have.
If we are dealing with tin(IV) ions, then we deduce the formula as follows:
The ions are Sn4+ and Cl-.
The charges encountered are (without regard to sign) 4 and 1. The LCD of 4 and 1 is 4. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Sn4+ we have 4 / 4 = 1
For Cl- we have 4 / 1 = 4
Thefore, the correct formula for the compound containing these ions is SnCl4. The name of this compound is tin(IV) chloride because it contains tin(IV) ions -- NOT because it has four chloride ions in the formula. Again, take note of this, because it is a misconception students sometimes have.
It happened that the number of anions (Cl-) in the above examples matched the Roman number, even though that is not what the Roman number indicates. This is because the anion had a charge of 1-, so it will take as many anions as there are positive charges on the cation to get a neutral formula. For an example of compounds where the number of anions is NOT equal to the Roman number, consider the following examples:
Example 5 What are the Stock names and chemical formulas of the two tin oxides?
Solution
As we have seen, tin forms the ions tin(II), which is Sn2+ and tin(IV) which is Sn4+. Oxygen forms only one ion -- the oxide ion is always encountered as O2-. One of the compounds will be named tin(II) oxide and the other will be named tin(IV) oxide.
For tin(II) oxide, the formula is determined as follows:
The ions are Sn2+ and O2-
The charges encountered are (without regard to sign) 2 and 2. The LCD of 2 and 2 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Sn2+ we have 2 / 2 = 1
For O2- we have 2 / 2 = 1
Thefore the formula of tin(II) oxide is SnO. The subscrips of 1 are understood and not written. Notice that even though this is tin(II) oxide, there is only one oxide ion in the formula. The Roman number always indicates the positive charge on the metal element, never the number of ions.
For tin(IV) oxide, the formula is determined as follows:
The ions are Sn4+ and O2-
The charges encountered are (without regard to sign) 4 and 2. The LCD of 4 and 2 is 4. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Sn4+ we have 4 / 4 = 1
For O2- we have 4 / 2 = 2
Theforme the formula of tin(IV) oxide is SnO2. The subscript of 1 on Sn is understood and not written. Note that tin(IV) oxide has only 2 oxide ions, not 4. The Roman number indicates the charge on the metal ion, not the number of ions.
More examples with Stock names:
Example 6 What is the Stock name of Cr2O3?
Solution
We must determine the charge on the metal ion in order to be able to write the Stock name, because the charge must be given in the name as a Roman number. We can use the fact that the formula must be electrically neutral to arrive at the charge of the chromium ion.
Letting XCr be the charge on chromium and XO be the charge on oxygen, electrical neutrality requires that the following equation be satisfied:
2 * XCr + 3 * XO = 0
Since oxygen only forms the O2- ion, we know that
XO = -2
Substituting the second equation in the first one gives
2 * XCr + 3 * (-2) = 0
Multiplying the 3 and the -2 gives
2 * XCr - 6 = 0
Transposing the -6 to the right hand side gives
2 * XCr = 6
And finally, dividing both sides by 2 gives
XCr = 3
Now we know which chromium ion this is. It is the chromium(III) ion. Therefore, the name is chromium(III) oxide.
Example 7 What is the Stock name of FeO?
Solution
We must determine the charge on the metal ion in order to be able to write the Stock name, because the charge must be given in the name as a Roman number. We can use the fact that the formula must be electrically neutral to arrive at the charge of the iron ion.
Letting XFe be the charge on iron and and XO be the chrage on oxygen, electrical neutrality requires that the following equation be safisfied:
XFe + XO = 0
Since oxygen only forms the O2- ion, we know that
XO = -2
Substituting the second equation in the first one gives
XFe - 2 = 0
Transposing the -2 to the right hand side gives
XFe = 2
Now we know which iron ion this is. It is the iron(II) ion. Therefore, the name is iron(II) oxide.
Example 8 What is the Stock name of Cu2O?
Solution
We must determine the charge on the metal ion in order to be able to write the Stock name, because the charge must be given in the name as a Roman number. We can use the fact that the formula must be electrically neutral to arrive at the charge of the copper ion.
Letting XCu be the charge on copper and XO be the charge on oxygen, electrical neutrality requires that the following equation be satisfied:
2 * XCu + XO = 0
Since oxygen only forms the O2- ion, we know that
XO = -2
Substituting the second equation in the first one gives
2 * XCu - 2 = 0
Transposing the -2 to the right hand side gives
2 * XCu = 2
Dividing both sides by 2 gives
XCu = 1
Now we know which copper ion this is. It is the copper(I) ion. Therefore, the name is copper(I) oxide.
Before leaving the subject of Stock names, there is one ion that I should mention because its formula is not quite what you would expect from its Stock name. Given the Stock name mercury(I) ion, you would probably be tempted to write Hg+ as the formula of the ion. But actually, the formula is Hg22+. Since the charge on this ion is 2+, you might be wondering why this is not the mercury(II) ion. It must be remembered that there are TWO mercury ions in this formula, giving a TOTAL charge of 2+. The charge on EACH mercury ion is 1+. The mercury(II) ion is Hg2+. The mercury(I) ion is polyatomic and the mercury(II) ion is monatomic. The total ion charge is 2+ in both cases, but the charge per ion is 1+ in the case of mercury(I) and 2+ in the case of mercury(II).
Consider the ions of manganese. Their names in both the Stock
system and IC / OUS system are given below:
| ION | Stock Name | IC / OUS Name |
|---|---|---|
| Mn2+ | Manganese(II) ion | Manganous ion |
| Mn3+ | Manganese(III) ion | Manganic ion |
| Mn4+ | Manganese(IV) ion | No IC / OUS name for this ion |
Another drawback to this system is that the IC / OUS name does not actually specify the charge of the ion. It only tells you whether you are dealing with the higher or the lower of the ion's two charges. For example, suppose I mention the cobaltous ion. This only tells you I am dealing with the lower of cobalt's two charges. If you don't know what charges of ion cobalt forms, you still don't know what the charge of the ion is. This can be a real problem, since there is no simple relationship between the location of a transition element in the periodic table and the charges of ion it will form. In the case of cobalt, the two charges are 2+ and 3+. Having been told this, you can now identify the cobaltous ion as Co2+. The cobaltic ion is, of course, Co3+.
The suffixes IC and OUS used in this system are attached to the end of either the English or the Latin name of the element. Whether Engligh or Latin is used depends on which language the element gets its symbol from. In the case of manganese (Mn) and cobalt (Co) the chemical symbol is taken from the English name, so the English name is used in the IC / OUS system. Thus we have the manganous ion and the manganic ion for manganese, and the cobaltous ion and cobaltic ion for cobalt. However, for tin, we don't say "tinous ion" and "tinic ion". The symbol for tin is Sn, which comes from the Latin word stannum. Thus we have the stannous ion (which is Sn2+) and the stannic ion (which is Sn4+). Again, notice that the OUS ending is applied to the ion of lower charge and the IC ending is applied to the ion of higher charge.
There is one exception to the rule that the suffixes IC and OUS are applied to the Latin name of the element when the chemical symbol is taken from Latin. Since the chemical symbol of mercury (Hg) is taken from the Latin name hydragyrum, you might expect its two ions to be named hydragyrous ion and hydragyric ion. This is not the case, however. The ions are named mercurous ion (Hg22+) and mercuric ion (Hg2+). Recall that back in the section on the Stock system, we saw that mercury did not follow the usual pattern with regard to the formula of one of its ions. Mercury(I) ion was Hg22+ rather than Hg+ as you might have expected. Now, in this section on IC / OUS names, you again see that mercury is an exception to the usual rule. The ions are named in Engligh, rather than Latin, as you might have expected.
Given below is a listing of some ions having more than one charge that
we will often deal with in this course:
| ELEMENT | IONS OBSERVED FOR THIS ELEMENT |
|---|---|
| Chromium | Cr2+ and Cr3+ |
| Cobalt | Co2+ and Co3+ |
| Copper | Cu+ and Cu2+ |
| Iron | Fe2+ and Fe3+ |
| Lead | Pb2+ and Pb4+ |
| Manganese | Mn2+, Mn3+ and Mn4+ |
| Tin | Sn2+ and Sn4+ |
Given the information from a table like that above, it is not difficult to work with the IC /OUS system. Some examples follow:
Example 9 What is the formula of chromous oxide?
Solution:
Since chromium forms the ions Cr2+ and Cr3+ and since the suffix -ous refers to the ion of lower charge, we know the chromous ion must be Cr2+. The oxide ion is O2-. We then proceed to work out the formula in the usual way.
The ions are Cr2+ and O2-. The charges encountered (without regard to sign) are 2 and 2. The LCD of 2 and 2 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Cr2+ we have 2 / 2 = 1
For O2- we have 2 / 2 = 1
Therefore, the formula of chromous oxide is CrO. The subscripts of 1 are understood and not written.
Example 10 What is the formula of cupric chloride?
Solution
Since copper forms the ions Cu+ and Cu2+ and the suffix -ic refers to the ion of higher charge, we know the cupric ion is Cu2+. The chloride ion is Cl-. We then proceed to work out the formula in the usual way.
The ions are Cu2+ and Cl-. The charges encountered (without regard to sign) are 2 and 1. The LCD of 2 and 1 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Cu2+ we have 2 / 2 = 1
For Cl- we have 2 / 1 = 2
Therefore, the formula of cupric chloride is CuCl2. The subscript of 1 on Cu is understood and not written.
Example 11 What is the name of Mn2O3 in the IC / OUS system?
Solution
We must determine what the charge of the manganese ion is in this compound so we can see whether it is the higher or lower of its two common charges. Recognizing that the formula must be electrically neutral, and letting XMn be the charge of the manganese ion and XO be the charge of the oxide ion, the following equation must be satisfied:
2 * XMn + 3 * XO = 0
Since oxygen only forms the O2- ion, we know that
XO = -2
Substituting this in the equation above, we have
2 * XMn + 3 * (-2) = 0
Multiplying the 3 and the -2 gives
2 * XMn -6 = 0
Tranposing the -6 to the right hand side gives
2 * XMn = 6
Finally, dividing both sides by 2 gives
XMn = 3
From the table presented earlier in these notes, we can see that Mn3+
is the manganic ion. Therefore the name of Mn2O3
is manganic oxide. We should note that Mn3+ is named as
the higher charge of the manganese ion, even though manganese is also sometimes
encountered as Mn4+. As was pointed out earlier, the IC
/ OUS system will only distinguish between two different charages.
In the case of manganese, the 4+ charge is relatively rare, and 2+ and
3+ are the more common charges. Therefore, it is the 2+ and 3+ charges
that are handled by the
IC / OUS system. This makes Mn3+ the ion of "higher"
charge.
Example 12 What is the name of PbO in the IC / OUS system?
Solution
We must determine the charge of the lead ion in this compound. We can then see if it is the higher or lower of the two charges. We know that the formula PbO must be electrically neutral. Letting XPb be the charge of the lead ion, and XO be the charge of the oxide ion, the following equation must be satisfied:
XPb + XO = 0
Since oxygen only forms the O2- ion, we know that
XO = -2
Substituting this in the above equation gives
XPb + (-2) = 0
or more simply
XPb - 2 = 0
Transposing the -2 to the right-hand side gives
XPb = 2
Since lead forms the ions Pb2+ and Pb4+ and since the suffix -ous refers to the lower charge, the Pb2+ ion must be the plumbous ion. Therefore the name of PbO is plumbous oxide.
So far, all the ions we have encountered have been made up of just one atom. We may have taken more than one ion, but each ion itself consisted of a single atom that had either gained or lost electrons to give it an electric charge. Sometimes, however, groups of atoms take on an electric charge. The single atom ions we have considered so far are called monatomic ions, and when groups of atoms take on a charge, we call them polyatomic ions. The atoms within a polyatomic ion are covalently bonded -- that is, they are held together by sharing electrons. In this regard, the polyatomic ion resembles a molecule, but a molecule would not have an electric charge. Thus, when the group of covalently bonded atoms has an electric charge, it is a polyatomic ion rather than a molecule.
Determining names and formulas for ionic compounds containing polyatomic
ions really isn't any more difficult than for those containing only monatomic
ions once you know the formuls and charges of the polyatomic ions.
There are tables in which you can look up the formulas and charges of the
common polyatomic ions. As we will soon see, there is a great deal
of order in how the names are assigned to the polyatomic ions. With
a knowledge of the rules, and a little practice, you will find that you
can quickly memorize the names of the polyatomic ions you regularly deal
with. A table of polyatomic ions appears on page 56 of your Hill
& Petrucci textbook. A Web version of this table is shown below.
| Some Common Polyatomic Ions | ||||
| Name | Formula | Example | ||
| Cations | ||||
| Ammonium ion | NH4+ | NH4Cl | ||
| Hydronium ion | H3O+ | None | ||
| Anions | ||||
| Acetate ion | C2H3O2- | NaC2H3O2 | ||
| Carbonate ion | CO32- | Li2CO3 | ||
| Hydrogen carbonate ion
(or bicarbonate ion) |
HCO3- | NaHCO3 | ||
| Hypochlorite ion | ClO- | Ca(ClO)2 | ||
| Chlorate ion | ClO3- | NaClO3 | ||
| Perchlorate ion | ClO4- | KClO4 | ||
| Chromate ion | CrO42- | K2CrO4 | ||
| Dichromate ion | Cr2O72- | (NH4)2Cr2O7 | ||
| Cyanate ion | OCN- | KOCN | ||
| Thiocyanate ion | SCN- | KSCN | ||
| Cyanide ion | CN- | KCN | ||
| Hydroxide ion | OH- | NaOH | ||
| Nitrite ion | NO2- | NaNO2 | ||
| Nitrate ion | NO3- | NaNO3 | ||
| Oxalate ion | C2O42- | CaC2O4 | ||
| Permanganate ion | MnO4- | KMnO4 | ||
| Phosphate ion | PO43- | Na3PO4 | ||
| Hydrogen phosphate ion
(or biphosphate ion) |
HPO42- | Na2HPO4 | ||
| Dihydrogen phosphate ion | H2PO4- | NaH2PO4 | ||
| Sulfite ion | SO32- | Na2SO3 | ||
| Hydrogen sulfite ion
(or bisulfite ion) |
HSO3- | NaHSO3 | ||
| Sulfate ion | SO42- | Na2SO4 | ||
| Hydrogen sulfate ion
(or bisulfate ion) |
HSO4- | NaHSO4 | ||
| Thiosulfate ion | S2O32- | Na2S2O3 | ||
Later, we shall learn the pattern behind the names of these ions, but for now, let's just use the above table to solve some more problems. In the examples that follow, the ionic compounds will contain at least one polyatomic ion.
Example 13 What is the formula of ammonium sulfate?
Solution
Ammonium and sulfate are both polyatomic ions. Looking in the table of polyatomic ions (see above or see page 56 in your textbook) we find that ammonium ion is NH4+ and sulfate ion is SO42-. Once we have have this information, we proceed as before:
The ions are NH4+ and SO42-. The charges encountered (without regard to sign) are 1 and 2. The LCD of 1 and 2 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For NH4+ we have 2 / 1 = 2
For SO42- we have 2 / 2 = 1
Therefore we must take the ammonium twice and the sulfate ion once. Let's temporarily show the 1's and write the formula as
(NH4)2(SO4)1
Since we don't write subscripts when they are equal to 1, we don't need the parentheses around the SO4 either. Now we can write the formula as
(NH4)2SO4
My reason for showing the subscript 1 at first was to emphasize that it is indeed 1. There is a temptation to conclude that the anion subscript is 4. However, the 4 is part of the SO4 group. This group is taken one time, NOT four times. Therefore, the anion subscript is 1 even though we see a 4 at the end of the formula. Note that when a polyatomic ion is taken more than once, it must be enclosed in parentheses. We see this with the ammonium ion, NH4+, which has been used two times in the formula.
Example 14 What is the formula of barium hydroxide?
Solution
Barium is not a polyatomic ion. Barium ions are monatomic and we can decide the charge by noting the location of barium in the periodic table. We find barium in group 2A, so we expect the barium ion to be Ba2+. Hydroxide, on the other hand, is a polyatomic ion, so we must either know what it is or look it up in a table of polyatomic ions. We can not look it up in the periodic table because hydroxide is not simply the anion of an element. It is composed of TWO elements. The hydroxide ion is OH-. Now that we know what the ions are, we can proceed as usual.
The ions are Ba2+ and OH-. The charges encountered (without regard to sign) are 2 and 1. The LCD of 2 and 1 is 2. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Ba2+ we have 2 / 2 = 1
For OH- we have 2 / 1 = 2
Therefore, the barium ion must be taken once, and the hydroxide ion must be taken twice. Remember that when a polyatomic ion is taken more than once, it must be enclosed in parentheses. Therefore, in our formula, we must enclose the hydroxide group, OH-, in parentheses.
The formula of barium hydroxide is Ba(OH)2. The subscript of 1 on barium is understood and not written. A common error on this problem is to write the formula as BaOH2. This is incorrect becasue it only takes the H atom twice and not the O atom. Both the O and H atoms in OH- must be taken twice. This is because OH- is a group and must be taken as a unit.
Example 15 What is the formula of ammonium nitride?
Solution
Ammonium is a polyatomic ion. You will find it listed in tables as NH4+. But nitride is a monatomic ion. Generally, if the anion ends in -ide, it is a monatomic ion rather than a polyatomic ion. The exceptions to this are hydroxide (OH-), cyanide (CN-) and peroxide (O22-). Since nitride is not one of these three exceptions, we conclude that it is monatomic. Therefore, we use the periodic table to decide on the charge of the nitride ion. The name nitride is derived from the name nitrogen. We find nitrogen in group 5A of the periodic table, so we expect the nitride ion to be N3-. Now that we know what the ions are, we proceed as usual.
The ions are NH4+ and N3-. The charges encountered (without regard to sign) are 1 and 3. The LCD of 1 and 3 is 3. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For NH4+ we have 3 / 1 = 3
For N3- we have 3 / 3 = 1
Therefore, the NH4+ ion must be taken 3 times, and the N3- ion must be taken 1 time. So the formula of ammonium nitride is (NH4)3N. The subscript of 1 on N is understood and not written.
Example 16 What is the formula of aluminum carbonate?
Solution
Aluminum will form monatomic ions. By the location of aluminum -- in group 3A of the periodic table -- we expect the aluminum ion to be Al3+. Carbonate is a polyatomic ion. A good clue is that it's name does not end in -ide. Recall that most anions ending in -ide are monatomic. All anions NOT ending in -ide are polyatomic. The carbonate ion is CO32-. Now that we know what the ions are, we can proceed as usual.
The ions are Al3+ and CO32-. The charges encountered (without regard to sign) are 3 and 2. The LCD of 3 and 2 is 6. The subscript of each ion is calculated by dividing the LCD by the ion charge.
For Al3+ we have 6 / 3 = 2
For CO32- we have 6 / 2 = 3
Therefore, the Al3+ ion must be taken 2 times and the CO32-
ion must be taken 3 times. So the formula of aluminum carbonate is
Al2(CO3)3.
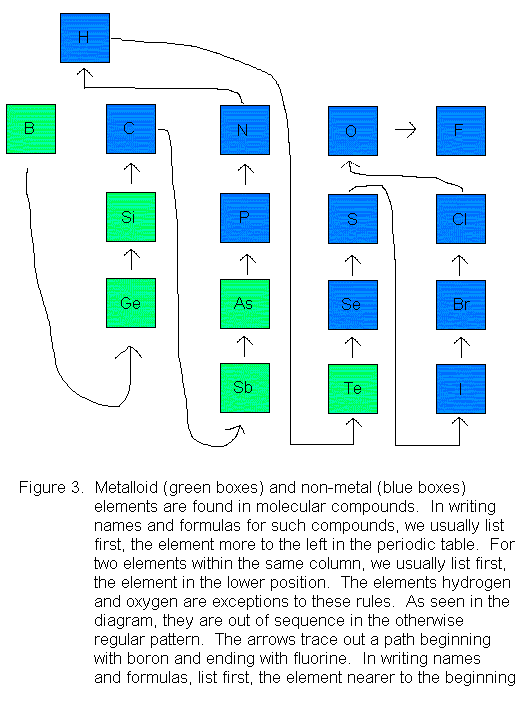
In general, we list first, the element on the left side of the periodic table, followed by the one on the right side. If the two elements in question fall within the same column, then we list the one at the bottom first, followed by the one at the top. Shown below is a diagram patterned after Figure 2.7 on page 50 in your Hill & Petrucci textbook. In this excerpt of the periodic table, only the non-metal and metalloid elements are shown, because metals are not associated with molecular compounds (again, see the table at the beginning of these notes).
In addition to knowing the order in which to list the elements, we
need to know how many atoms are present for each element. Where ionic
compounds were concerned, we could figure this out from the electric charges
of the ions. But since the atoms in molecular compounds are regarded
as neutral, there could be any number of atoms present. We therefore
need so specify the number with a Greek prefix. For example, consider
the oxides of nitrogen. Listed below are several nitrogen oxides,
by name and chemical formula.
| NAME
. |
FORMULA |
| nitrogen monoxide | NO
. |
| nitrogen dioxide
. |
NO2 |
| dinitrogen monoxide
. |
N2O |
| dinitrogen tetroxide
. |
N2O4 |
| dinitrogen pentoxide
. |
N2O5 |
Notice that nitrogen is mentioned before oxygen in these names and formulas. This is in keeping with the rule illustrated in Figure 3. The Greek prefixes are used to indicate the number of atoms of each element in these formulas. Note that the prefix mono- is understood and not included explicitly on the first element. It is used on the second element, however. Thus, we name NO nitrogen monoxide, not mononitrogen monoxide or nitrogen oxide. A substance that uses the mono- prefix that you might be more familiar with is the poisonous gas CO. We call it carbon monoxide, not monocarbon monoxide or carbon oxide.
As you can see, if you know the meaning of the Greek prefixes, it is
a simple matter to deduce the name from the formula or the formula from
the name. Shown below are the Greek prefixes for the numbers from
1 through 10.
| PREFIX
. |
NUMBER |
| mono-
. |
1 |
| di-
. |
2 |
| tri-
. |
3 |
| tetra-
. |
4 |
| penta-
. |
5 |
| hexa-
. |
6 |
| hepta-
. |
7 |
| octa-
. |
8 |
| nona-
. |
9 |
| deca-
. |
10 |
Sometimes there are minor variations in spelling of these prefixes for ease of pronunciation. A compound with a single oxygen atom has a name that ends with -monoxide, not -monooxide (example: NO). With 4 oxygen atoms, the name ends with -tetroxide rather than -tetraoxide (example: N2O4) and with 5, -pentoxide rathen than -pentaoxide (example: N2O5).
Example 17
What is the name and formula of the compound with molecules that contain 10 atoms of oxygen and 4 atoms of phosphorous?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter phsophorous (P) before oxygen (O). Therefore, phosphorous will be listed first in both the name and the formula. There are 4 phosphorous atoms so we use the prefix tetra-, and there are 10 oxygen atoms, so we use the prefix deca-, but shortened to dec- for ease of pronunciation. So the name is tetraphosphorous decoxide, and the formula is P4O10.
Example 18
What is the name and formula of the compound with molecules that contain 2 chlorine atoms and 7 oxygen atoms?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter chlorine (Cl) before oxygen (O). Therefore, chlorine will be listed first in both the name and the formula. There are 2 chlorine atoms so we use the prefix di-, and there are 7 oxygen atoms, so we use the prefix hepta-, but shortened to hept- for ease of pronunciation. So the name is dichlorine heptoxide and the formula is Cl2O7.
Example 19
What is the name and formula of the compound with molecules that contain 3 chlorine atoms and 1 nitrogen atom?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter nitrogen (N) before chlorine (Cl). Therefore, nitrogen will be listed first in both the name and the formula. There is 1 atom of nitrogen, but the prefix mono- is omitted for the first element. There are 3 chlorine atoms, and the prefix for 3 is tri-. So the name is nitrogen trichloride, and the formula is NCl3.
Example 20
There is a compound of hydrogen and nitrogen with molecules that contain 3 hydrogen atoms and 1 nitrogen atom. This compound has been known since ancient times, and as a result, is usually referred to by its common name -- ammonia -- rather than being given a systematic name. However, if it were given a systematic name, what would it be? And what is its formula?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter nitrogen (N) before hydrogen (H). Therefore, nitrogen will be listed first in both the name and the formula. There is 1 atom of nitrogen, but the prefix mono- is omitted for the first element. There are 3 hydrogen atoms, and the prefix for 3 is tri-. So the systematic name is nitrogen trihydride. The formula is NH3.
Example 21
The gas known as methane consists of molecules that each contain 1 atom of carbon and 4 atoms of hydrogen. What is the systematic name of this compound, and what is its formula?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter carbon (C) before hydrogen (H). Therefore, carbon will be listed first in both the name and the formula. There is 1 atom of carbon, but the prefix mono- is omitted for the first element. There are 4 hydrogen atoms, and the prefix for 4 is tetra-. So the systematic name is carbon tetrahydride. The formula is CH4.
Note: The name carbon tetrahydride is seldom used, and methane is generally regarded as a correct chemical name for this compound. Still the problem makes a worthwile exercise for our purposes.
Example 22
Water is hardly ever given a systematic chemical name. But according to the rules of nomenclature we have been learning for binary molecular compounds, what would its systematic name be?
Solution
Practically everyone knows the formula of water is H2O, so we already know in which order the elements should be listed. Still, you might want to look at Figure 3 and confirm that this is the correct order (as opposed to OH2). In a water molecule, there are 2 hydrogen atoms, for which we use the prefix di-, and 1 oxygen atom, for which we use the prefix mono-, but shortened to mon- for ease of pronunciation. The systematic name of water -- if anyone used it -- would be dihydrogen monoxide.
Example 23
What is the name and formula of the compound with molecules that contain 2 atoms of fluorine and 1 atom of oxygen?
Solution
First, we need to know in which order to list the elements. By reference to Figure 3, we see that starting from the boron end, we encounter oxygen (O) before fluorine (F). Therefore, oxygen will be listed first in both the name and the formula. There is 1 atom of oxygen, but the prefix mono- is omitted for the first element. There are 2 fluorine atoms, for which we use the prefix di-. Therefore, the name is oxygen difluoride, and the formula is OF2.
Binary acids are only named as such when they are dissolved in water.
Otherwise, they are named as "ordinary" binary molecular compounds.
For example, the halogens (fluorine, chlorine, bromine, and iodine) all
form binary compounds with hydrogen. When pure, they are named as
follows:
| FORMULA
. |
BINARY NAME |
| HF
. |
hydrogen fluoride |
| HCl
. |
hydrogen chloride |
| HBr
. |
hydrogen bromide |
| HI
. |
hydrogen iodide |
As you may recall, the prefix mono- is omitted for the first element, but it is usually included for the second element. Perhaps you're wondering why it has been omitted here on the second element as well. The reason is that these are the only binary compounds that these elements form with hydrogen. The prefixes are used to distinguish one molecular compound from another when the same two elements form more than one compound. Therefore, their use is not necessary here.
Each of the compounds in the above table, when dissolved in water, produces
a solution that is acidic. So when these compounds are dissolved
in water rather than in the pure state, they are named as binary acids
rather than "ordinary" binary compounds. The rules for naming binary
acids are as follows:
The binary compounds in the table above have the following acid names
when dissolved in water:
| FORMULA
. |
ACID NAME |
| HF
. |
hydrofluoric acid |
| HCl
. |
hydrochloric acid |
| HBr
. |
hydrobromic acid |
| HI
. |
hydroiodic acid |
In the table above, notice that the part of the name in bold type is common to all of the acid names. The above acids just use the stem name of the element with which the hydrogen combines. But now consider the following example.
Example 24
The element sulfur forms a binary compound with hydrogen that behaves as an acid when dissolved in water. This compound, consisting of molecules containing 1 atom of sulfur and 2 atoms of hydrogen, is the only binary compound formed by these two elements. Based on this information, determine the formula of this compound, its name as a pure compound, and its name when dissolved in water.
Solution
First, we need to know in which order to list the elements. By
reference to figure 3, we see that starting from the boron end, we encounter
hydrogen (H) before sulfur (S). Therefore, hydrogen will be listed
first in both the binary name the the formula. Since this is the
only
binary compound of these two elements, no prefixes will be needed, so the
binary name is hydrogen sulfide. The formula is H2S.
When named as a binary acid, the form must be hydro__________ic
acid. In this case, we don't take just the stem of the element's
name -- we take the whole name (sulfur). Putting sulfur on the blank
line above (and making it all one word) we have hydrosulfuric
acid.
HNO3 is nitric acid (more oxygen atoms)
HNO2 is nitrous
acid
(fewer oxygen
atoms)
H2SO4 is sulfuric acid (more oxygen atoms)
H2SO3 is sulfurous
acid
(fewer oxygen atoms)
H3PO4 is phosphoric acid (more oxygen atoms)
H3PO3 is phosphorous acid (fewer oxygen atoms)
Sometimes hydrogen and oxygen may form more than two oxoacids with the same element. In that case, a prefix is also needed, because the suffixes -ous and -ic can only distinguish between two possibilities. The element chlorine provides an example:
HClO4 is perchloric acid (most oxygen atoms)
HClO3 is chloric acid (more oxygen atoms)
HClO2 is chlorous acid (less oxygen atoms)
HClO is hypochlorous acid (least oxygen atoms)
There is a simple correspondence between the names of the oxoacids and the names of the oxoanions you encountered earlier in these notes, and in Table 2.4 on page 56 of your Hill & Petrucci text. Although these acids are regarded as molecular compounds, they do ionize in water, and in so doing, mimic ionic compounds. The acids ionize by splitting into one or more H+ ions and an oxoanion, as determined by the formula of the acid. Shown below are the ionizations of all the oxoacids we have looked at so far. Some of these acids do not spontaneously lose all of their hydrogens as shown here (unless a base is added to help the process along). We will learn later about the difference between strong and weak aicds. For now, however, I'm removing ALL the hydrogens (where possible) from the acid to obtain the polyatomic anion. Our goal here is to compare the name of the acid to the name of the anion it contains -- and recognize a pattern.
HNO3(aq) ---------->
H+(aq) + NO3-(aq)
nitric acid
nitrate ion
HNO2(aq) ---------->
H+(aq) + NO2-(aq)
nitrous acid
nitrite ion
H2SO4(aq) ---------->
2H+(aq) + SO42-(aq)
sulfuric acid
sulfate ion
H2SO3(aq) ---------->
2H+(aq) + SO32-(aq)
sulfurous acid
sulfite ion
H3PO4(aq) ---------->
3H+(aq) + PO43-(aq)
phosphoric acid
phosphate ion
H3PO3(aq) ---------->
2H+(aq) + HPO32-(aq)
Note: the remaining H is not ionizable
phosphorous acid
hydrogen phosphite ion
or
biphosphite ion
HClO4(aq) ---------->
H+(aq) + ClO4-(aq)
perchloric
acid
perchlorate
ion
HClO3(aq) ---------->
H+(aq) + ClO3-(aq)
chloric acid
chlorate ion
HClO2(aq) ---------->
H+(aq) + ClO2-(aq)
chlorous acid
chlorite ion
HClO(aq) ----------> H+(aq)
+ ClO-(aq)
hypochlorous
acid
hypochlorite
ion
Notice the pattern which has emerged. If the acid's name ends with the suffix -ic, the oxoanion it contains ends with the suffix -ate. Likewise, if the acid's name ends with the suffix -ous, the oxoanion it contains ends with the suffix -ite. Notice also that since each hydrogen ion carries one unit of positive charge (H+), the number of hydrogens in the acid will be equal to the number of negative charges on the anion it contains. This means that if you know your oxoanions, you can predict the formulas of oxoacids. The following example illustrates this.
Example 25
What is the formula of carbonic acid?
Solution
From the preceeding discussion, we reason that carbonic acid would be related to the carbonate ion. By reference to Table 2.4 on page 56 in your Hill & Petrucci text (or the equivalent list earlier in these notes) we see that the carbonate ion is CO32-. Since there are two negative charges, it should require two H+ ions to cancel out all the charge and make a neutral molecule. Therefore, the formula of carbonic acid is expected to be H2CO3.
In the above example, we went from a name to a formula. We can also go from a formula to a name, as shown in the following example.
Example 26
What is the name of H2CrO4?
Solution
This formula contains hydrogen, oxygen, and another
element, so we recognize it as a ternary acid (or oxoacid). Looking
at the part of the formula that comes after the hydrogen, we can find the
oxoanion we need to look up in Table 2.4. Since there are 2 hydrogens
in this formula, the CrO4 group must have two units of negative
charge. That is, the ion we should look for is CrO42-.
According to Table 2.4, the name of this ion is chromate.
From the chromate ion,
we should get chromic acid.
HCO3- is the hydrogen carbonate ion or bicarbonate ion
HSO3- is the hydrogen sulfite ion or bisulfite ion
HPO42- is the hydrogen phosphate ion or biphosphate ion
HS- is the hydrogen sulfide ion or bisulfide ion
In the last example above, note the importance of using the word "ion" in naming HS-. The name "hydrogen sulfide" (without the word ion following it) refers to the neutral compound H2S, not the ion HS-.
Note also that the naming method that uses the bi- prefix only applies to those acid anions with a single hydrogen. For example, the ion H2PO4- can be referred to as "dihydrogen phosphate ion", but there is no name for this ion in the bi- system, because it has 2 hydrogens instead of only 1.
The acid anions form compounds with cations, just as any other anion does. These compounds are named in the same way as other ionic compounds -- the positive ion is named first and the negative ion is named last. Examples:
NaHCO3 is sodium hydrogen carbonate or sodium bicarbonate
Ca(H2PO4)2 is calcium dihydrogen phosphate
Al(HSO3)3 is aluminum hydrogen sulfite or aluminum bisulfite
Na2H2PO4 is sodium dihydrogen phosphate
Students sometimes have trouble writing formulas for ionic compounds containing acid anions, perhaps because the names tend to be longer and more complicated looking. The following are some examples of writing formulas for these compounds.
Example 27
What is the formula of ammonium bisulfite?
Solution
The ions are ammonium, which is NH4+, and bisulfite, which is HSO3-. The charges encountered (without regard to sign) are 1 and 1. The LCD of 1 and 1 is 1. The subscript on each ion is calculated by dividing the LCD by the ion charge.
For NH4+ we have 1 / 1 = 1
For HSO3- we have 1 / 1 = 1
Therefore, each ion must be taken once, and the formula is NH4HSO3.
Example 28
What is the formula of calcium hydrogen sulfate?
Solution
Although this name has 3 words, it only has 2 parts: the cation name and the anion name. In this compound, the cation is calcium ion and the anion is hydrogen sulfate ion. Once the ions are identified, we proceed as usual.
The ions are Ca2+ and HSO4-. The charges encountered (without regard to sign) are 2 and 1. The LCD of 2 and 1 is 2. The subscript on each ion is calculated by dividing the LCD by the ion charge.
For Ca2+ we have 2 / 2 = 1
For HSO4- we have 2 / 1 = 2
Therefore, the Ca2+ ion should be taken 1 time and the HSO4- ion should be taken 2 times. The formula is Ca(HSO4)2. The subscript of 1 on Ca is understood and not written.
Example 29
What is the formula of magnesium bisulfide?
Solution
The magnesium ion is Mg2+ and the bisulfide ion is HS-. Now that the ions have been identified, we proceed as before.
The ions are Mg2+ and HS-. The charges encountered (without regard to sign) are 2 and 1. The LCD of 2 and 1 is 2. The subscript on each ion is calculated by dividing the LCD by the ion charge.
For Mg2+ we have 2 / 2 = 1
For HS- we have 2 / 1 = 2
Therefore the Mg2+ ion must be taken
1 time and the HS- ion must be taken 2 times. The formula
is Mg(HS)2.
The following are some examples dealing with names and formulas of hydrated salts.
Example 30
What is the name of CoCl2 . 6H2O?
Solution
We must name both the main part of the formula (CoCl2) and the attached water (6H2O). Since cobalt is a transition metal, we must account for the fact that it forms more than one charge of ion. The cobalt ion is known both as Co2+, which is cobaltous ion or cobalt(II) ion, and Co3+, which is cobaltic ion or cobalt(III) ion. We must decide which ion we have in this compound. The chloride ion has 1 unit of negative charge, that is, Cl-. We have 2 of these ions, so the total negative charge is -2. There is only 1 cobalt ion in this formula, so it must contribute 2 units of positive charge to make a neutral formula. Therefore, we have the Co2+ ion in this formula. We can also show this algebraically:
Let XCo = charge on the cobalt ion
Let XCl = charge on the chloride ion
The H2O is electrically neutral, so we need not consider it in the calculation. Looking at the CoCl2 part of the formula, electrical neutrality requires that the following equation be satisfied:
XCo + 2XCl = 0
But we know that the chloride ion is Cl-, so we can make the following variable assignment:
XCl = -1
We can then substitute this in the previous equation to get
XCo + 2 (-1) = 0
which can be written
XCo - 2 = 0
Then transposing the -2 to the other side of the equation, we have
XCo = +2
So CoCl2 is cobaltous chloride in the -ic / -ous system and cobalt(II) chloride in the Stock system. It has 6 waters attached to it, and the Greek prefix for 6 is hexa-. Therefore the complete name is cobaltous chloride hexahydrate in the -ic / -ous system and cobalt(II) chloride hexahydrate in the Stock system.
Example 31
What is the formula of calcium chloride dihydrate?
Solution
The calcium ion is Ca2+ and the chloride ion is Cl-. By the LCD method you have learned previously, we can show that the salt formula is CaCl2. The "dihydrate" at the end of the name tells us that there are 2 water molecules attached to it, so the complete formula is CaCl2 . 2H2O.
Example 32
What is the name of Na2CO3 . 10H2O?
Solution
The salt part of the formula is sodium carbonate.
We have previously learned how to come up with such names. The new
feature here is the attached water. We see 10 water molecules attached.
The Greek prefix for 10 is deca-. Therefore the complete name for
this hydrated salt is sodium carbonate decahydrate.